2020 Volume 128 Issue 3 Pages 93-111
2020 Volume 128 Issue 3 Pages 93-111
We examined fragmentary human skeletal remains from Escalon Cave near Surigao City, northeastern Mindanao, the Philippines, with respect to the morphology of bones and teeth, radiocarbon dating, and mitochondrial DNA (mtDNA) haplogroup. These remains contained parts of the left temporal bone, the right humerus, the right femur, the upper right first and second premolars, and the first molar. They are presumed to belong to an early-middle adult male, whom we named the Escalon Man. Using the femur sample, we estimated the 14C date of the individual at 2692 ± 39 years BP (uncalibrated). His stature, estimated from the maximum femur length, was about 170 cm—this makes him much taller than the Aeta and the Mamanwa, who are indigenous negrito hunter-gatherers of the Philippines. The femur is sturdy and shows well-developed muscle markings. Numerous narrow grooves on the humerus were found in close proximity to each other, but we could not prove whether these are artificial cut marks made by humans with sharp-edged tools or scratches from some non-human entity. The mesiodistal crown diameters of the molar and premolars are large and resemble those of native Oceanians. Analysis of mtDNA haplogroup was carried out using a DNA sample extracted from a molar. A next-generation sequencer was used to determine the nucleotide sequences of the mtDNA genome. The results indicated that the Escalon Man belongs to the haplogroup E1a1a, which is known to be the marker of Austronesian-speaking agriculturist populations that originated in Taiwan and spread southwards through the Philippines to the Western Pacific, since about 4000 BP. Thus, the Escalon Man was likely a member of the late Neolithic or early Metal Age agriculturist peoples who settled in northeastern Mindanao and who may be the ancestors of the territory’s present-day occupants, such as the Manobo.
Apart from the recent finding of the archaic hominin Homo luzonensis in northern Luzon (Mijares et al., 2010; Détroit et al., 2019), few studies have been conducted on prehistoric skeletal remains of modern humans (Homo sapiens) in the Philippines (Figure 1). These studies include that of the Tabon Man (14C dated at c. 22000–24000 BP) and the Dujong Man (3730 BP), both of which were from Palawan (Fox, 1970; Dizon, 2002; Détroit et al., 2004). Skeletal remains were found in the San Lorenzo III Shell Midden in northern Luzon (14C dated at 1815 BP) (Ramirez and Tanaka, 2013), and the remains of Panhutongan were discovered in northeastern Mindanao (dated at 140 AD) (Bauzon and Dizon, 1997).

Map A shows the locations of hominid sites in the Philippines. The white square in map B corresponds to the whole area of map C. The Escalon Cave (★) is located about 100 m upstream of the Cagniog–Ceniza Road bridge (♦), where the Cagniog–Ceniza Road crosses the Escalon River.
These studies also deal with archaeological materials such as pottery and stone and shell tools, and their 14C dates were obtained from charcoal samples at the site (Fox, 1970). In the present study, however, no such archaeological materials were available, except some suspicious implements (see below).
Of the remains described in the aforementioned studies, only the Tabon Man was of Pleistocene origin. This might indicate that Homo sapiens arrived in the Philippine Islands more than 10000 years BP. It is tempting to speculate that the Tabon Man may be an ancestor of the Batak people, a negrito group of the island of Palawan.
Negritos, such as the Aeta of Luzon and the Mamanwa of Mindanao, are indigenous hunter-gatherer people of the Philippines. Recent genetic studies using mitochondrial (mt) and nuclear DNA data confirm that the negrito population originated in the Pleistocene era, some 20000–40000 years ago (HUGO Pan-Asian SNP Consortium, 2009; Delfin et al., 2014; Jinam et al., 2017). While these studies suggest that the negrito population of the Philippines originates from Sundaland, the Mamanwa of northeastern Mindanao may have slightly different origins. Based on classic genetic markers, they may have arrived in Mindanao slightly earlier than other negrito groups, from some parts of Wallacea (Omoto, 1985, 1989). Since Escalon Cave happens to be situated in the territory of the present-day Mamanwa ethnic group, the present study began with the expectation of finding some clues to the origins of the Mamanwa.
Discovery of the Escalon ManA cave containing human skeletal remains was discovered in the 1980s by a local treasure hunter who was excavating calcified sediments along the Escalon River. This river drains north into Bilang-Bilang Bay in Cagniog Village, approximately 6 km to the southeast of Surigao City in the province of Surigao del Norte, Caraga Region (XIII), northeastern Mindanao, the Philippines (Almeda, 2017) (Figure 1). The cave is situated at the left bank of Escalon River, about 100 m upstream from the Cagniog-Ceniza Road bridge, and opens to a steep cliff about 12 m above the riverbed. It is approximately 8 m wide, 5 m high, and 5 m deep, faces northeast, and has an entrance about 5 m wide and 4 m high (Figure 2).

Entrance of the Escalon Cave, mostly covered by trees and bushes (left panel), and the east wall and floor inside the cave (right panel) from where the human bones were dug out. The vertical scale is 1.0 m. Long tree roots are seen on the floor.
The cave was unnamed and was given no scholarly attention until it was revealed that there were human bones inside. Because the bones were found in a cliffside cave along Escalon River, the cave was dubbed Escalon Cave by Almeda and other scholars. Further, the human skeletal remains from the cave were named, en bloc, the Escalon Man.
Almeda and Louca, who served as the volunteer curator of Surigaonon Heritage Center (SHC), investigated Escalon Cave in 2003. They noted that most of the calcified floor deposits and flowstones (travertine) had been extracted by the treasure hunter (Louca, 2003). Nevertheless, the precipitation and deposition of calcite within the cave corroborated the treasure hunter’s assertion that human bones had been embedded within flowstone floor deposits, close to the east wall (Louca, 2003; Almeda, 2017) (Figure 2, Figure 3).

Structure of the Escalon Cave floor, rearranged from Louca (2003). 1, carbonate deposits probably contained artifacts; 2, the flowstone (travertine) which contained the human bones; 3, the thin mud layer on the bedrock floor; 4, the limestone bedrock; 5, black organic matter and shells found on the floor in 2003; 6, fragments of deposits scattered on the floor. Human bones were probably found under the east wall near the cave’s entrance (⋆).
In 2005, L.E.B. met K.O. in Tokyo, told him about the findings at Escalon Cave, and proposed that they carry out a joint Filipino–Japanese collaborative study of the skeletal remains. K.O. agreed, and soon travelled to Surigao City to meet Almeda, view the remains (which were being held by the SHC), and visit the cave. K.O. decided that the remains deserved to be the focus of deeper, integrated research, including chronological, genetic, and physical anthropological studies. L.E.B., F.A.A., and K.O. then discussed how to conduct such studies and finally proposed various Japanese collaborators who agreed to join in the research.
Incidentally, we noted two unusual archaeological objects in the SHC’s showcase: a large limestone spearhead and a small earthenware jar. These objects had also been discovered by the treasure hunter in Escalon Cave; however, there was no evidence that they were associated with the human remains. Moreover, the shape of these objects seemed rather artificial. Therefore, we asked two authorities in Philippine and Pacific archaeology—Professor Kazuhiko Tanaka (Tsurumi University, Japan) and Professor Michiko Intoh (National Museum of Ethnology, Japan)—to determine the objects’ veracity by reviewing photographs of them. However, to the best of these experts’ knowledge, the objects’ form bore no resemblance to any archaeological objects in the region. Therefore, we decided not to include these objects in the present study.
Samples of the Escalon Man were transported, with the permission of the National Museum of the Philippines in Manila, to the National Museum of Nature and Science (NMNS) in Tokyo, Japan, in 2008 and 2009. H.B. was the head of the NMNS’s Department of Anthropology at that time, and he carried out morphological studies of the samples with K.S. E.K. examined the teeth and M.Y. oversaw the radiocarbon dating. Studies of the genetic origins of the Escalon Man using mtDNA haplogroups were carried out by H.K.-K. and K.S., and by T.K. and N.A. Then K.O. prepared the manuscript for publication.
The bones and teeth of the Escalon Man were found encased in flowstones and/or calcified matrices in Escalon Cave (Figure 3). The thickness of these associated flowstones varied between approximately 0.5 and 5 cm. Indeed, some bone shafts were broken and exposed because of fractures caused by these stones.
Flowstones and associated calcified matrices were carefully removed from bones by H.B. and K.S. in the NMNS laboratory (Figure 4). These preparations revealed that the remains included a part of the left temporal bone, fragmentary shafts of the right humerus and right femur, the first and second premolars, and the first molar from the right maxilla (Figure 4, Figure 5, Figure 6). In addition, the alveolar part of the right maxilla (containing three embedded teeth) was also identified in the flowstone; however, this bone was too thin and fragile to be removed. Thus, only the teeth were extracted from the maxilla for further study.

The mastoid process and the adjacent region of the left temporal bone (A, lateral view; B, inferoposterior view). The digastric fossa is marked (↑). The maxilla fragment, holding three teeth, is embedded in the flowstone (C, inferomedial view).

The right femur fragment and the flowstone which covered the femur. Posterior views.
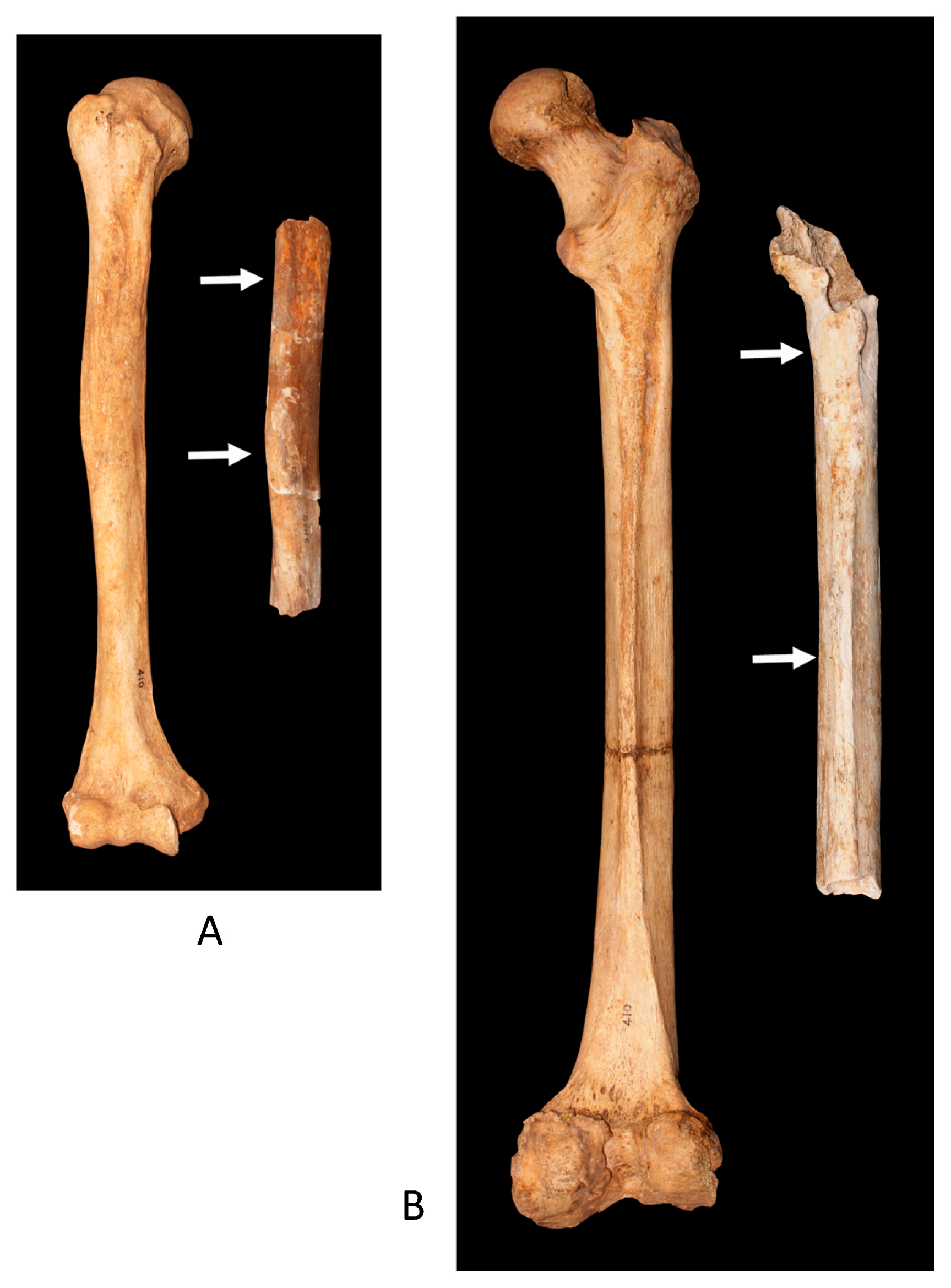
Preserved portions of the right humerus (A, frontal view) and the right femur fragment (B, posterior view), compared with those of an exceptionally tall, stout Japanese Mesolithic/Neolithic Jomon male from the Wakaumi shell midden site, whose stature is estimated at 170 cm. These elements are almost equal in length. Arrows indicate the positions of cross-sections in Figure 9 and Figure 11.
The Escalon Man’s bones and teeth were moderately hard and somewhat fragile. The original surfaces of these specimens were light yellowish-brown in color, even though the newly broken parts were more or less white. The teeth remained largely intact, with the exception of small breaks and some moderate attrition. Although the surface of original bones remained generally intact, small to medium-sized shallow holes were found in several places on the humerus and the femur, and these holes sometimes connected with one another to form larger holes (Figure 7, Figure 8). These holes generated sharp edges and somewhat rough inner surfaces. Although the holes could be the result of either soil and water erosion or insect activity, Ortner (2003) asserts that they were likely not caused by either pathological lesions or animal bites.

The humerus fragment from the Escalon Man. The shaft has a slight anterolateral flex at the lower end of the deltoid tuberosity, fitting almost to the midshaft (⋆). An X-ray photograph shows the thickness of the cortex.

The right femur fragment of the Escalon Man. The shaft is bowed forward (○) and the linea aspera projects high, forming a pilaster (⋆). A longitudinal groove along the lesser trochanter is seen in medial view (↑). An X-ray photograph shows the thickness of the cortex.
Numerous small prominences that corresponded exactly to the positions and shapes of the holes on these bones were observed on the inner surface of the flowstones which had been detached from these bones. The available data suggest that the taphonomic process involved the initial decay of human remains to bones, followed by the development of the calcified matrix that covered the floor of the cave (Figure 3). Then, the bones were covered with mud, and numerous holes appeared on this surface because of an unknown reaction. Calcium carbonate from the bedrock crystallized onto the bone surfaces to form the flowstone (travertine) and calcified matrices between 0.5 and 5 cm thick.
Description and comparison of bonesBecause the bones and teeth were found together in a relatively narrow area of the cave and their morphological features are similar to each other, it seemed reasonable to infer that they belonged to one individual, probably an adult male.
Measurements of bones and teeth were made according to the methods proposed by Martin (Bräuer and Knussman, 1988; Baba, 1991) and Fujita (1995), respectively. Data were then compared with those from several East Asian, Oceanian, and Pacific populations from the Paleolithic era to the present day (Table 1, Table 2, Table 3, Table 4).
| Measurement items with Martin’s no. | (2620 ± 39 BP) | Paleolithic | Mesolithic/Neolithic | Neolithic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Philippines | Japan | China | Japan | China | ||||||||||
| Escalon Man | Minatogawa 11 | Upper Cave2 | Wakaumi3 | Tsukumo4 | Yang Shao Tsun5 | |||||||||
| male | male | males | male | males | females | males | f + R69emales | |||||||
| M | N | Mean | SD | N | Mean | SD | N | Mean | N | Mean | ||||
| Humerus | ||||||||||||||
| 1 Maximum length | 300–340* | 287 | 313 | 15 | 291.5 | 12.4 | 16 | 266.2 | 9.3 | 2 | 332 | 4 | ||
| 2 Total length | 296–336** | 287 | 12 | 286.2 | 10.6 | 14 | 262.5 | 7.7 | 269.8 | |||||
| 5 Maximum diameter of mid-shaft | 23 | 20.5 | 26 | 20 | 23.9 | 1.84 | 25 | 20.4 | 2.0 | 4 | 23.5 | 5 | 18.4 | |
| 6 Minimum diameter of mid-shaft | 18 | 15.5 | 18 | 20 | 17.5 | 1.44 | 25 | 14.1 | 1.3 | 4 | 17.7 | 5 | 13.6 | |
| 6/5 Cross-section index | 78.3 | 75.6 | 69.2 | 20 | 72.7 | 3.8 | 25 | 69 | 5.3 | 4 | 75.4 | 5 | 73.9 | |
| 7 Minimum circumference | 63 | 58 | 64 | 20 | 65.2 | 3.7 | 23 | 55.2 | 2.8 | 51.1 | ||||
| 7a Circumference at mid-shaft | 70 | 61 | 71 | 19 | 70.1 | 5.2 | 25 | 58.6 | 4.2 | |||||
| 7/1 Robusticity index | 18.5–21.0 | 20.2 | 20.5 | 15 | 22.8 | 1.3 | 13 | 20.5 | 1.3 | |||||
| Femur | ||||||||||||||
| 1 Maximum length | 450–510* | 398 | 461 | 13 | 418.2 | 20 | 16 | 382.9 | 14.9 | 4 | 458.7 | 5 | ||
| 2 Physiological length | 446–506** | 393 | 458 | 13 | 414.2 | 20.1 | 16 | 377.8 | 13.4 | 375 | ||||
| 9a Maximum diameter of upper shaft | 33 | 29# | 32.0 | 33 | 21 | 30.5 | 1.8 | 25 | 28.3 | 1.1 | 4 | 33.0 | 6 | 24.6 |
| 10a Minimum diameter of upper shaft | 24 | 23# | 30.2 | 24 | 21 | 24.2 | 1.4 | 25 | 21.6 | 1.6 | 4 | 25.5 | 6 | 19.9 |
| 10a/9a Platymeric index | 72.7 | 79.3 | 94.3## | 72.7 | 21 | 79.5 | 5.9 | 25 | 76.6 | 5.2 | 4 | 78.3 | 6 | 80.7 |
| 6 Sagittal diameter of mid-shaft | 32 | 26.5 | 32.0 | 34 | 19 | 29.3 | 1.6 | 26 | 25.0 | 1.7 | 4 | 31.2 | 6 | 24.3 |
| 7 Transvers diaeter of mid-shaft | 26 | 26.0 | 28.1 | 28 | 19 | 25.5 | 1.5 | 26 | 24.0 | 1.2 | 4 | 28.0 | 6 | 20.6 |
| 6/7 Pilasteric index | 123.1 | 101.9 | 113.9## | 121.4 | 19 | 114.6 | 8.3 | 26 | 103.9 | 7.5 | 4 | 110.1 | 6 | 118.1 |
| 8 Circumference at mid-shaft | 90 | 83 | 91 | 19 | 86.8 | 3.4 | 26 | 77.4 | 3.8 | 69.4 | ||||
| 8/2 Robusticity index | 17.8–20.2 | 21.1 | 19.9 | 13 | 21.1 | 0.9 | 16 | 20.5 | 0.9 | 18.6 | ||||
| Martin’s no. | Neolithic | Modern to Recent | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lapita related, Oceania | Japan | Taiwan | Philippine | ||||||||||||||||||
| Mokapu6 | Sigatoka7 | Tonga8 | Ahu Tepeu9 | Watom10 | Kinai11 | Fukien Formosan12 | Luzon negrito13 | ||||||||||||||
| males | males | females | males | males | males | females | males | females | males | females | |||||||||||
| N | Mean | SD | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | Mean | Mean | N | Mean | N | Mean | |
| Humerus | |||||||||||||||||||||
| 1 | 47 | 317.8 | 12.9 | 3 | 332.0 | 6 | 307.7 | 7 | 324 | 8 | 318.8 | 2 | 337.5* | 294.2 | 273.9 | 313.4 | 294.0 | ||||
| 2 | 44 | 312.9 | 13.5 | 2 | 333.5** | 289.8 | 269.5 | 308.3 | 289.4 | 12 | 276.0 | 9 | 269.8 | ||||||||
| 5 | 68 | 23.1 | 1.7 | 9 | 24.7 | 12 | 22.8 | 8 | 21.3 | 3 | 23.0 | 22.3 | 19.5 | 22.4 | 19.9 | 12 | 19.6 | 12 | 18.4 | ||
| 6 | 68 | 17.4 | 1.2 | 9 | 19 | 12 | 16.1 | 8 | 15.4 | 3 | 18.7 | 17.4 | 14.6 | 17.1 | 15.2 | 12 | 13.7 | 12 | 13.6 | ||
| 6/5 | 68 | 75.6 | 5.1 | 77.0 | 70.7 | 8 | 72.4 | 3 | 81.3 | 78.1 | 75.1 | 76.9 | 75.4 | 12 | 69.7 | 12 | 73.9 | ||||
| 7 | 13 | 66.8 | 14 | 60.2 | 2 | 64.5 | 64.6 | 54.6 | 62.0 | 55.0 | 12 | 53.2 | 9 | 51.1 | |||||||
| 7a | 65 | 66.3 | 4.2 | 9 | 70.2 | 12 | 64.3 | 66.3 | 57.2 | 64.3 | 57.3 | ||||||||||
| 7/1 | 20.1 | 19.6 | 2 | 20.7* | 21.8 | 19.9 | 19.8 | 18.8 | |||||||||||||
| Femur | |||||||||||||||||||||
| 1 | 49 | 440.3 | 18.6 | 8 | 451.6 | 4 | 435.8 | 4 | 461 | 11 | 452.2 | 1 | 452* | 413.7 | 382.3 | 435.7 | 399.7 | ||||
| 2 | 50 | 436.8 | 18.6 | 5 | 445.6 | 3 | 436.0 | 11 | 448.4 | 1 | 448** | 409.9 | 377.6 | 432 | 395 | 11 | 380.5 | 10 | 375 | ||
| 9a | 65 | 33.4 | 2.4 | 14 | 30.5# | 20 | 30.2# | 8 | 32# | 11 | 32.1 | 4 | 33.0# | 29.3 | 27.3 | 29.6 | 25.7 | 11 | 25.6 | 10 | 24.6 |
| 10a | 65 | 23.8 | 1.6 | 14 | 25.1# | 20 | 23.5# | 8 | 27# | 11 | 22.9 | 4 | 28.5# | 25.2 | 22.3 | 23.7 | 21.0 | 11 | 20.3 | 10 | 19.9 |
| 10a/9a | 65 | 71.6 | 4.1 | 82.2 | 77.8 | 8 | 84.8 | 11 | 71.3 | 4 | 84.8 | 86.3 | 82.0 | 80.9 | 83.3 | 11 | 79.4 | 10 | 80.7 | ||
| 6 | 64 | 29.7 | 2.1 | 14 | 30.3 | 18 | 28.6 | 11 | 31 | 11 | 29.6 | 4 | 29.0 | 27.2 | 23.3 | 27.0 | 23.4 | 11 | 25.2 | 10 | 24.3 |
| 7 | 64 | 25.0 | 1.7 | 14 | 26.1 | 18 | 24.9 | 11 | 27 | 11 | 25.9 | 4 | 24.3 | 25.3 | 23.1 | 25.6 | 22.9 | 11 | 20.3 | 10 | 20.6 |
| 6/7 | 64 | 118.5 | 8.8 | 115.8 | 114.5 | 11 | 116.9 | 11 | 114.0 | 4 | 119.3 | 108.1 | 101.1 | 104.1 | 102.4 | 11 | 117.2 | 10 | 118.1 | ||
| 8 | 59 | 85.9 | 5.5 | 14 | 90.2 | 18 | 85.4 | 4 | 88 | 83.2 | 74.2 | 84.3 | 73.9 | 11 | 71.1 | 10 | 69.4 | ||||
| 8/2 | 49 | 19.2 | 0.9 | 1 | 19.6 | 20.3 | 19.7 | 19.6 | 18.1 | 11 | 18.7 | 10 | 18.6 | ||||||||
Except for the values of Ahu Tepeu and Watom and the femur lengths of Minatogawa, all values are from the right side.
| Tooth | Mesiodistal diameter | Buccolingual diameter | Crown height | Root length | Total length |
|---|---|---|---|---|---|
| Maxillary first premolar | 7.82 | 9.89 | 7.56 | 11.63 | 19.19 |
| Maxillary second premolar | 7.52 | 9.83 | 6.70 | 13.66 | 20.36 |
| Maxillary first molar | 11.81 | 12.58 | 6.14 | 12.83 | 18.97 |
| Maxillary first premolar | Maxillary second premolar | Maxillary first molar | ||||||
|---|---|---|---|---|---|---|---|---|
| MD | BL | MD | BL | MD | BL | |||
| Palau1 | 7.98 | 10.13 | Fiji2 | 7.65 | 10.63 | Escalon6 | 11.81 | 12.58 |
| Fiji2 | 7.96 | 10.65 | Palau1 | 7.62 | 9.95 | Fiji2 | 11.56 | 12.73 |
| Samoa2 | 7.88 | 10.14 | Samoa2 | 7.61 | 10.23 | New Guinea3 | 11.41 | 12.68 |
| Escalon6 | 7.82 | 9.89 | Escalon6 | 7.52 | 9.83 | Palau1 | 11.34 | 12.54 |
| New Guinea3 | 7.81 | 10.68 | New Guinea3 | 7.14 | 10.70 | Australia4 | 11.2 | 12.55 |
| Australia4 | 7.63 | 10.25 | Australia4 | 7.13 | 10.23 | Samoa2 | 11.06 | 12.16 |
| Negrito5 | 7.47 | 9.48 | Negrito5 | 7.10 | 9.29 | Negrito5 | 11.02 | 11.59 |
MD, mesiodistal diameter; BL, buccolingual diameter. Data sources of populations are as follows:
| Sex | MD | BL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P1/M1 | P2 | P2/M1 | M1 | P1 | P1/M1 | P2 | P2/M1 | M1 | ||
| Escalon6 | Male | 7.82 | 0.66 | 7.52 | 0.64 | 11.81 | 9.89 | 0.79 | 9.83 | 0.78 | 12.58 |
| Philippines1 | Male | 5.86 | 0.68 | 6.56 | 0.65 | 10.02 | 9.42 | 0.85 | 9.28 | 0.84 | 11.11 |
| Japanese2 | Male | 7.51 | 0.71 | 7.05 | 0.67 | 10.60 | 9.73 | 0.82 | 9.48 | 0.80 | 11.86 |
| Negrito3 | Male | 7.47 | 0.68 | 7.10 | 0.64 | 11.02 | 9.48 | 0.82 | 9.29 | 0.80 | 11.59 |
| New Britain4 | Male | 7.10 | 0.64 | 6.10 | 0.55 | 11.10 | 9.10 | 0.79 | 9.10 | 0.79 | 11.50 |
| Western Australia5 | Male | 7.38 | 0.64 | 7.03 | 0.60 | 11.62 | 10.30 | 0.81 | 10.30 | 0.81 | 12.78 |
Data sources of populations are as follows:
A part of the left temporal bone encompassing the region between the petrous pyramid and the occipitomastoid suture, including the asterion, was preserved. However, some of the petrous pyramid remained encased in the flowstone and was too difficult to remove from the fragile temporal bone (Figure 4). No evidence for the closure of sutures can be ascertained. The squama temporalis and most of the tympanic bone have been lost. The bone’s surface is stained yellowish brown, with visible whitish fractures.
The mastoid process of the temporal bone is large, and the digastric groove (mastoid notch) is wide and deep, measuring 6 mm and 8 mm, respectively. This indicates that this individual was likely male. In general, muscle markings around the mastoid process are clear and there is no evidence of degenerative changes or enthesophytes. There is also no sign of exostosis on the external auditory canal, which is often regarded as characteristic among fishermen and fisherwomen who regularly dive in water. The presence of clear muscle marks on the temporal bone is indicative of well-developed neck muscles, such as the sternocleidomastoideus and splenius capitis.
HumerusA fragmental shaft of the humerus measuring 162 mm in length and encompassing the proximal and central parts of the bone was preserved (Figure 6). Although the surface of this bone was originally covered by flowstone between 0.5 and 1 cm thick, and the lower end was slightly weathered, it is likely to have been broken and exposed to the flowstone before it was removed from the cave floor by treasure hunters. There are innumerable holes, probably due to erosion, on the outer surface of this bone (Figure 7).
Although only about half of the whole length of the bone remains, its maximum length was roughly estimated as 300–340 mm, based on the comparison with about 150 intact Japanese humeri from Mesolithic/Neolithic Jomon to modern periods, stored at the NMNS (Kajigayama and Baba, 1999) (Figure 5, Table 1). Since the maximum length of the humerus was not shown in some of the comparable specimens, we also estimated an alternative total length (physiological length) of 296–336 mm by subtracting 4 mm from the maximum length, based on the difference between maximum and total lengths of the humerus samples in the collection of recent Japanese from Kinai (Miyamoto, 1925; Hirai and Tabata, 1928) (Table 1). These measurements are close to, or larger than, male mean values from East Asian and Oceanian populations and much larger than those for females.
The Escalon Man’s humeral shaft appears to be sturdy and somewhat flat (Figure 9). It is slightly compressed anteromedially–posterolaterally at the lower end of the deltoid tuberosity, at a height almost equal to the mid-point (Figure 6, Figure 7). Muscle scarring on the shaft is fairly developed, but not very rough (Figure 9). Judging from its size—especially its maximum length (300–340 mm) and the maximum and minimum diameters of the midshaft (23 and 18 mm, respectively)—the Escalon Man’s humerus appears to be much larger than those of Aetan and Mamanwan males (Genet-Varcin, 1951; Omoto, 1985, 1989). The Escalon Man’s humerus is also larger than those of the Minatogawa Man from the Paleolithic Okinawa (Baba and Endo, 1982), the male Jomon samples from Mesolithic/Neolithic Japan (Kiyono and Hirai, 1928a; Imamura, 1996), and modern Japanese and Fukien Formosan, or Taiwanese populations originating from Fukien province, China (Hsu, 1949).
The dimensions of the Escalon Man’s humerus resemble those of Neolithic Chinese people from Yang Shao Tsun (Black, 1925) and Pacific Islanders, such as the Sigatoka people of Fiji (Pietrusewsky et al., 1994) and the Mokapu of Hawaii (Snow, 1974) (Table 1). At the same time, the robusticity index of the Escalon humerus (18.5–21.0%) does not differ markedly from that of Oceanian, Fukien Formosan, or Paleolithic Minatogawa males; however, it is smaller than that of modern Japanese males from Kinai and much smaller than that of Jomon males, who possessed short and thick humeri (Table 1).
The shaft of the Escalon humerus has clear muscle marks, including crests of the greater (for the pectoralis major) and lesser (for the latissimus dorsi and the teres major) tubercles (Figure 9). The anterior ridge of the deltoid tuberosity (for the deltoideus) projects high above the shaft to form an angle, while the anteromedial surface around the midshaft (for the brachialis) is wide and flat, as is often the case in Jomon males (Baba, 1988).
Results show that the cross-sectional humeral midshaft index of the Escalon Man is average (78.3%); this is equivalent to the values observed in modern Fukien Formosan males and Japanese males from Kinai, and larger and less flat than what has been observed in Jomon males, who possessed flattened shafts with marked muscle scars (Figure 9). The anterolateral shaft flexion seen in the Escalon humerus (Figure 7) is also a characteristic feature of the Paleolithic Minatogawa people of Okinawa and the Jomon people (Baba, 1988), but is rare in modern Japanese people. These humeral features suggest that the Escalon Man was a male with arms sturdier than most of the comparative specimens. The presence of numerous shallow grooves on the surface of the Escalon Man’s humeral bone, just above the deltoid tuberosity and running perpendicular to the long axis, represent an interesting finding; these grooves are described below in detail (Figure 10).

Cross-sections of the humerus of the Escalon Man compared with a Jomon male from the Wakaumi shell midden site and a recent Japanese male (Dokkyo University School of Medicine, DUSM81). Muscle markings (1, for the pectoralis major; 2, the latissimus dorsi; 3, the teres major; 4, the deltoideus; 5, for the brachialis) are clear and the midshaft is flattened in both the Escalon Man and the Jomon male.

Transverse grooves on the lateral and anterior surfaces of the right humerus fragment of the Escalon Man. Molds of these grooves are present on the inner surface of the flowstone fragment in A. Some grooves were enlarged in B. A minute white square in A corresponds to the white square in C and whole area of D. Scanning electron microscopy images in C and D revealed that the surface of the grooves was eroded, which obscures the cause of grooves.
A part of the right femur measuring 281 mm was preserved from the lesser trochanter to the lower shaft (Figure 6). The other sections of this bone were likely crushed when the bone was removed from the cave floor. This femur bone was originally covered by the flowstone, which was later removed in the laboratory (Figure 5). The surface of this bone is stained pale yellowish brown and is lighter in color than the humerus (Figure 7, Figure 8). There are numerous shallow small to medium-sized holes on the anterior, medial, and lateral surface, along with many corresponding prominences on the inner surface of the flowstone—as with the Escalon Man’s humerus.
We found that at the posterior surface of the lower end of the femur shaft, the lateral and medial lips of the linea aspera are still parallel with each other and do not show any signs of separation; furthermore, the lower shaft shows no increase in transverse diameter (Figure 6). According to Steele and McKern (1969), the length from the midpoint of the lesser trochanter to the most proximal extension of the popliteal surface, at the point where the medial and lateral supracondylar lines become parallel below the linea aspera, is 56.1% of the total (maximum) length, with an SD of 2.5%. In the Escalon Man’s femur, the length from the midpoint of the lesser trochanter to the lowest point of the linea aspera of the shaft is 247 mm. If the lowest point of the Escalon Man’s femur corresponds to the most proximal point (as per Steele and McKern, 1969), we estimate the maximum length of the Escalon Man’s femur at 440 mm with mean SD of 11 mm. However, as the lowest point in the Escalon Man’s femur most likely sits at least 1 cm, or probably 2–3 cm, higher than the point defined by Steele and McKern (1969), the length defined by these authors is probably at least 1 cm longer than the above assumption. Thus, we tentatively fix this length at 260–280 mm and estimated the maximum length (divided by 0.561) as 463–499 mm, with a mean SD of 11.6–12.5 mm. Finally, the maximum length of the Escalon Man’s femur was roughly 450–510 mm (Table 1). The physiological length might be 4 mm smaller, as in the humerus. These values are equal to or considerably larger than those of the males and significantly larger than those of the females, as represented in Table 1. The femur’s upper shaft is very flat and shows anterolateral–posteromedial compression (Figure 9). The bone appears to exhibit moderate anterior bowing and strong torsion, as can be determined from the remaining section (Figure 8).
The linea aspera of this femur is projected into a high and wide pilaster (Figure 8, Figure 11). At the proximal end, the medial lip of the linea aspera elongates into a well-marked pectineal line, and the lateral lip continues into a well-developed gluteal tuberosity (Figure 8). The lesser trochanter of this femur is extremely large, measuring 26 mm in length and 15 mm in width with moderate enthesophytes. A shallow but clear longitudinal groove (65 mm in length and 8 mm in width) is present, and it extends from the medial to the lesser trochanter.
The size of the Escalon Man’s femur—with its maximum length (450–510 mm), sagittal and transverse midshaft diameters (34 and 28 mm, respectively), and the maximum and minimum diameters of the upper shaft (33 and 24 mm, respectively)—suggests that he was much larger than either Aetan or Paleolithic Okinawan (Minatogawa) males, larger than Mesolithic/Neolithic Jomonese and modern Fukien Formosan males, and equivalent in size to Neolithic Chinese from Yang Shao Tsun and Lapita Oceanian males (Table 1).
Although the robusticity index of the Escalon Man’s femur (17.8–20.2%) is not high, this bone is sturdy, as evidenced by its absolute thickness and well-developed muscle scars (Figure 8, Figure 11). The formation of a high and thick pilaster, for example, indicates that the quadriceps and adductor muscles were very well developed.

Cross-sections of the femur of the Escalon Man compared with a Jomon male from Wakaumi shell midden site and a recent Japanese male (DUSM81). In the Escalon Man and the Jomon male, the upper shaft is flattened and the linea aspera is projected, forming a pilaster (★). In the Escalon Man, the long axis of the upper shaft cross-section (broken line) is steep, indicating strong torsion of the shaft.
Furthermore, the Escalon Man’s massive lesser trochanter and clearly developed longitudinal groove strongly implies the presence of a well-developed iliopsoas muscle. These findings may indicate that the Escalon Man had large and very powerful thighs and legs. Thus, as the Escalon Man was tall and considerably sturdy, with proportionately more powerful thighs and legs than his arms, it is likely that he engaged in ground-based activities, such as agriculture and/or hunting and gathering.
Identifying the Escalon Man via skeletal morphologyAs discussed above, the Escalon Man’s temporal bone, humerus, and femur are large with well-developed muscle attachments. Three teeth were also extracted from the maxilla embedded in the flowstone near the temporal bone; thus, it is reasonable to assume that these remains belong to a single, fully adult male.
We estimate the height of the Escalon Man at either c. 1661–1809 mm or c. 1694–1823 mm, using estimates of maximum femur length and formulae provided by Fujii (1960) for Japanese males and Trotter and Gleser (1952) for East Asian males, respectively. Thus, he may have been 170 cm tall or taller when he died.
Estimating the Escalon Man’s age at his time of death is somewhat difficult, since his livelihood was not clear. As discussed below, the Escalon Man exhibited only slight signs of tooth wear, which correspond to a young or sub-adult age in hunter-gatherers and an early-middle adult age for agriculturalists. However, the developments of the Escalon Man’s muscle attachment areas are fair or marked, which indicates that he may have been an early-middle or late-middle adult. Thus, it is assumed that he was an early-middle adult and that his livelihood may have involved agricultural activities.
The Escalon Man had very sturdy thighs and legs and considerably sturdy arms, and did not show external auditory exostosis, which is indicative of exposure to cold water. It is supposed that he may not be a fisherman who habitually rowed a boat or dove into the sea but likely worked in agriculture. In conclusion, the Escalon Man might have been a tall, early middle-aged farmer.
The presence of scratches or cut marksAs mentioned previously, numerous narrow grooves are present on the Escalon Man’s humerus: there are 5 on the lateral surface and 10 on the posterior surface (Figure 7, Figure 10). Most of these grooves comprised single lines and are straight and rather wide with cuneiform cross-sections. Some are thin and shallow and consist of double lines separated by about 1 mm. It is noteworthy that these double-lined grooves are only seen on the lateral surface of the humerus and there are no signs of subsequent healing. The color inside these grooves is the same as those of other surfaces, and these surfaces show numerous prominences that are similar to those on the inner surface of the flowstones, which were initially attached to the bone. It seems likely, therefore, that these marks were made probably around or after the time of death, and there is no doubt that they were made before the bones were covered by flowstones.
The grooves on the Escalon Man’s humerus are almost parallel with each other. They are perpendicular to the longitudinal axis of the humerus and vertical to the external surface (Figure 10). Most of these grooves appear very sharp with smooth and concave inner surfaces, when viewed with the naked eye and with a magnifying glass. However, when these marks were imaged with a scanning electron microscope (SEM), it was found that the surfaces of these grooves are in fact rough, probably due to erosion.
It appears that these grooves are neither deep cuts (often made during fights) nor shaving marks (usually indicative of cannibalism) nor the result of animal bites (Suzuki, 1956; Baba et al., 1987; White, 1992; Ortner, 2003). It is possible that they represent scratches made by trampling animals. However, we do not discount the possibility that they are man-made, because intentional bone modification in the Early Holocene has been reported from Palawan, the Philippines (Lara et al., 2013). It is unfortunate that no systematic way exists to determine the cause of this phenomenon in this specimen.
Three tooth samples were extracted from the maxillary fragment covered by the flowstone and made available for study (Figure 4, Figure 12). They included a maxillary first premolar, a maxillary second premolar, and a maxillary first molar. Measurements of these teeth are shown in Table 2.

Maxillary right first and second premolars, as well as the first molar of the Escalon Man. Abbreviations: M1, first molar; P1, first premolar; P2, second premolar; Lin, lingual; Buc, buccal; Mes, mesial; Dis, distal; Occ, occlusal.
The crown of this tooth was relatively large (Table 2, Figure 12) and exhibited typical angular curvature in its mesiobuccal and distobuccal corners, from an occlusal perspective. The occlusal surface was moderately worn, and one dentine spot, 1.0 mm in diameter, was exposed on the buccal cusp tip. The presence of an H-type occlusal groove pattern could not be ascertained in this case due to enamel attrition, even though the crown height of this tooth was relatively short compared with the size of the whole crown—also due to occlusal attrition. This tooth had a single root that was relatively short, thick, and flattened in a buccolingual (BL) direction; no longitudinal root groove was evident on the distal surface, and the distal curvature of the root tip was weak.
Maxillary right second premolarThe crown of this tooth was relatively large but was nevertheless slightly smaller than that of the first premolar because of its smaller mesiodistal (MD) diameter (Table 2, Figure 12). In this case, the BL diameter was almost the same as that of the first premolar, indicating that the second premolar had an elongated shape, from an occlusal perspective. The mesiobuccal corner of the buccal cusp of this tooth was also rounded, the occlusal surface was moderately worn, and a spot of dentine 0.5 mm in diameter was exposed at the buccal cusp tip. The H-type occlusal groove pattern was also clearer in this tooth than in the first premolar; the single root of this tooth was relatively short and thick, but longer than in the first premolar. This tooth was also flattened in a BL direction and any root tip distal curvature, if present, was slight.
Maxillary right first molarThe crown of this tooth was relatively large and bore typical four cusps (Table 2, Figure 12). The mesiobuccal corner of this tooth crown, however, was chipped—this may have affected the MD diameter measurements. Attrition of the occlusal surface was also moderate, and a spot of dentine 1.5 mm in diameter was exposed at the tip of the mesiolingual cusp, 1.0 mm in diameter at the mesiobuccal cusp and 0.5 mm in diameter at the distobuccal cusp. At the same time, no dentine was exposed at the tip of the distolingual cusp. The crown height was measured in this case from the mesiobuccal cusp, even though it may be shorter (between 1.0 and 2.0 mm) compared to the original tooth due to occlusal attrition. The pattern of the occlusal groove was also obscured because of attrition; there was no visible oblique ridge wear, and there was no trace of Carabelli’s cusp on the lingual surface. In addition, the presence of a fifth cusp between the mesiobuccal and mesiolingual cusps on this tooth could not be clearly ascertained, although a clear enamel extension was present on the buccal surface. This tooth had a typical three-root configuration, although the tips of the mesiobuccal and lingual roots have likely been chipped, and the central portion of the distolingual root featured a horizontal crack.
Comparative study of dental remainsSeveral comparative studies among Pacific populations have focused on the size of their teeth (Kieser, 1990). For example, Kanazawa et al. (2000) provided data for the average MD diameters of 14 tooth samples from each of 23 Pacific populations culled from the literature. The average MD and BL diameters of the three teeth dealt with in this paper are taken from the literature (Table 3).
Although it appears, at first glance, that figures for indigenous negrito populations’ teeth size are remarkably small, the data from Hanihara (1990)—which is an important case in the literature—only provides data for the Aeta people of Luzon, who are well known for their notably small body size. In contrast, other local negrito groups, such as the Mamanwa of Mindanao, are not quite so small as the Aeta (Omoto, 1989). Thus, to find out whether small tooth size is a general feature of Philippine negrito groups, scholars must examine the diversity of tooth sizes among various other Negritos.
Table 3 indicates that the Escalon Man had relatively large teeth, like those of Pacific peoples. Only three populations exhibit larger average MD diameters in the maxillary first and (7.82 mm) second premolars (7.52 mm) than the Escalon Man. In contrast, the diameters of the Escalon Man’s BL premolars (9.89 mm for the maxillary first premolar and 9.83 mm for the maxillary second premolar) are smaller than those found in most other populations. Similarly, the MD diameter of the Escalon Man’s first molar is the largest among all available data in the literature, and the BL diameter of this tooth is the fourth largest in the literature.
Matsumura (1995) has shown that the sizes of MD and BL diameters in premolars, relative to the first molar (i.e. P1/M1, P2/M1), varies between different Asian-Pacific populations. These ratios in Pacific people seem to be slightly smaller than those seen in populations from Japan and the Philippines, because the former possess relatively large first molars (Table 4). Although the sample size is small in most cases, simple comparisons of the sizes of these teeth suggest that the Escalon Man’s teeth are more similar to those of Pacific peoples than those of Asian peoples.
As mentioned previously, the literature is unclear on whether the small teeth of the Aeta should be considered a common feature of the negrito population in general. Unfortunately, data regarding the size of other negrito populations’ teeth have not been available until recently. In the context of this study, the most important data are those for the Mamanwa, the indigenous people of northeastern Mindanao whose territory includes the Escalon Cave site.
Estimating the age of the Escalon Man at death based on tooth wearTooth wear or dental attrition rates of the three teeth described above were as follows: P1, point exposure of dentine on the buccal cusp and broad facets of wear on enamel; P2, apical exposure on the buccal cusp and polished lingual cusp; and M1, point or small circular exposure on the mesiolingual, mesiobuccal, and distobuccal cusps. According to Lovejoy (1985), who presented functional attrition stages of the teeth corresponding to ages in Amerind (Libben, hunter-gatherers) in a series from A (no wear) to H (severe wear), these wear spots or facets represent P1, Phase C (age 18–22 years); P2, Phase B2 (age 16–20 years); and M1, Phase between C (age 18–22 years) and D (age 20–24 years).
However, these estimates do not match the our observations of the Escalon Man’s bones. The Escalon Man’s bones show clear, and sometimes strong developments in various muscle attachment areas. In addition, Fujita (1993) and Fujita and Ogura (2009) applied Tochihara’s (1957) method of scoring dental attrition rates on a scale of 1 to 8 for their comparative study of Jomon (hunter-gatherers), Yayoi (agriculturalist), and early modern Japanese peoples’ teeth. The attrition scores of Jomon hunter-gathers were ≥4 among early middle-aged males and ≥5 in late middle-aged males. The attrition scores of Yayoi agriculturalists were ≤3 in young adults and 4 in early middle-aged males. The attrition scores of early modern Japanese people were ≥2 in early middle-aged males and ≥3 in late middle-aged males. In comparison, the Escalon Man’s tooth wear scores were ≤4—i.e. his tooth wear is comparable to that of early middle-aged Yayoi agriculturalist males. Among Jomon hunter-gathers, an attrition score of ≤4 does not fit well with early middle-age and never fits late middle-aged males. Thus, it is reasonable to suppose that the Escalon Man was most likely an early middle-aged male agriculturalist.
An additional sample: dental impressions from a Mamanwan maleThanks to Dr. Jiji J. Miranda of Surigao City, we were able to obtain a plaster model of a dental impression of an adult Mamanwan male. According to Dr. Miranda, he was 23 years of age, 158 cm tall, and weighed 67 kg—thus, he was larger than the average adult Aeta male (Omoto, 1989). This dental impression was congenitally missing two maxillary third molars, but 30 other teeth were present and the dental arch shape was found to be generally normal.
The MD and BL diameters of these teeth were measured, and these data were then compared with 21 dental impression models of Aeta males housed in the University Museum of the University of Tokyo (Hanihara, 1990). The tooth size of the present Mamanwan sample exceeded almost all the teeth of the Aeta sample. The tooth wear observed on the Mamanwan sample’s enamel surface was moderate, and no dentine exposure was observed. Tooth diameters of the Mamanwa, in comparison with other Asian-Pacific populations reported thus far, suggested that they were nearly as large as Oceanic populations. The Escalon Man’s teeth are of a similar size, but a single sample is inadequate for a full and definite discussion of the phylogenetic relationship between the Escalon Man and the Mamanwa (Kanazawa et al., 2015).
To date the Escalon Man’s remains, radiocarbon dating was performed on the femur sample (ESM-1G). Gelatinization was conducted at 90°C to extract gelatin (Longin, 1971; Yoneda et al., 2002). The concentration of carbon and nitrogen in gelatin was measured with a Flash 2000 Elemental Analyzer (Thermo Fisher Scientific, Germany). Purified gelatin (0.25 mg) was weighed in a tin cup and measured with laboratory standards for correction. After evacuation, 2.5 mg of collagen was combusted into CO2 in a vacuum line (Minagawa et al., 1984), and CO2 was then reduced to graphite with H2 and iron powder catalysis for radiocarbon dating (Kitagawa et al., 1993). Graphite was measured for radiocarbon abundance by accelerator mass spectrometry at the Micro Analysis Laboratory (MALT), The University of Tokyo (Matsuzaki et al., 2007). The conventional radiocarbon age (years BP) was shown to 1 SD (Stuiver and Polach, 1977).
Table 5 shows the results of the elemental analysis and radiocarbon dating process. Unfortunately, the atomic ratio of carbon and nitrogen (C/N ratio) in the collagen was 3.8— higher than the normal range of 2.9–3.6 (DeNiro, 1985). This suggests a possible contamination and/or alteration of collagen. Because a C/N ratio of 3.8 can be caused by contamination of exogenous organic matter, the radiocarbon age of the sample—2620 ± 39 years BP—should be confirmed by reference to additional archaeological context from the site and/or other methods. We report this radiocarbon date to provide a frame of reference for the Escalon Man’s antiquity; however, a more precise resampling of material and a thorough re-evaluation of the sample’s archaeological context is needed to date him more accurately.
| sample | %C | %N | atomic C/N | conventional 14C age | Lab. code | Remarks |
|---|---|---|---|---|---|---|
| ESM-1G | 42.7 | 13.0 | 3.8 | 2620 ± 39 BP | MTC-13799 | Altered C/N ratio |
The first upper molar tooth of the right side was used for mitochondrial DNA (mtDNA) analysis. We followed the protocol set out by Adachi et al. (2011) for DNA extraction and analysis of amplified product-length polymorphism (APLP), and used the six-plex primer set M and N to tentatively identify the Escalon Man’s mtDNA haplogroup.
In addition to the APLP analysis, we applied next-generation sequencing (NGS) technology and mtDNA capture methods to determine the Escalon Man’s mtDNA haplogroup. The protocol set out by Shinoda et al. (2017) was used to prepare the DNA libraries, and a total of six DNA libraries were prepared (Supplementary Table 1). To efficiently investigate endogenous ancient human mtDNA, the libraries were enriched using the methods laid out by Maricic et al. (2010). The enriched libraries were then sequenced on Illumina MiSeq software (MiSeq Reagent Kit, 150 or 300 cycles). Raw sequence reads were processed and classified as single-nucleotide polymorphisms (SNPs) and haplogroups based on the protocol set out by Matsumura et al. (2018).
We investigated both the degree of terminal cytosine to thymine (C-to-T) misincorporation using postmortem damage (PMD) tools (Skoglund et al., 2014) and read length distribution. These are key elements in determining DNA sequences derived from ancient samples. We estimated the samples’ contamination frequency using Schmutzi software (Renaud et al., 2015) alongside the procedure set out by Kanzawa-Kiriyama et al. (2017).
Mitochondrial genome analysisIt was not possible to identify the Escalon Man’s mtDNA haplogroup by APLP analysis because the extracted DNA exhibited a high level of degradation. We therefore used NGS and the mtDNA capture methods to determine the Escalon Man’s mtDNA haplotype from complete mitochondrial genome sequences. We obtained 3303 unique mtDNA fragments (Supplementary Table 1) covering most of the mitochondrial genome (Supplementary Figure 1). Short mtDNA fragments and a high frequency of terminal C-to-T misincorporations indicate that those reads originated from the Escalon Man, and not from contaminations by modern human DNA (Supplementary Figure 2, Supplementary Figure 3). According to the changes in the mtDNA coding sites, the Escalon Man’s mtDNA haplogroup was classified as E1a1a1a (Table 6). Contamination frequency was estimated to 6–13% (Supplementary Table 2a, b). Therefore, we conclude that most of the mtDNA was derived from the Escalon Man sample, and his mitochondrial DNA haplogroup is E1a1a1a.
| Sample ID | Haplogroup | SNPs or indels against rCRS |
|---|---|---|
| Escalon Man | E1a1a1a | A73G, A263G, T310TC, T489C, A750G, A1438G, A2706G, T3027C, G3705A, T4248C, G4491A, A4769G, T6620C, C7028T, G7598A, A8701G, T8843C, A8860G, T9540C, A10398G, C10400T, C10834T, T10873C, G11719A, C12705T, T13254C, C13626T, T14577C, T14783C, G15043A, G15301A, A15326G, C16223T, C16291T, T16362C, G16390A, T16519C |
Note: Underlined mutations have low depth (£2) but these are consistent with mutation of haplogroup E1a1a1a.
The results of previous studies (e.g. Hill et al., 2007) show that haplogroup E is a common indigenous haplogroup in the islands of Southeast Asia (ISEA). This group is common among indigenous Taiwanese people and is prevalent among the Austronesian-speaking populations outside of China; however, it is almost absent across mainland China and the Pacific region. Furthermore, haplogroup E has been regarded as a potential marker of early Holocene populations who expanded from the center of the ISEA to neighboring regions (Hill et al., 2007; Soares et al., 2008).
However, judging from its demographic distribution and time depth, some of the haplogroup’s subclades spread subsequently from Taiwan to the ISEA—haplogroup E1a1a is one of its candidates (Tabbada et al., 2010). This sub-haplogroup exhibits less diversity among Philippine and Sulawesi populations than among indigenous Taiwanese people (Soares et al., 2008). Thus, although haplogroup E may be a marker of post-glacial expansion, subclades within this haplogroup such as E1a1a reflect the impact of later population events. Among the subclade E1a1a, sub-haplogroup E1a1a1a appears to be associated with the Austronesian population expansion that spread since the Neolithic period, which is probably related to the expansion and development of agriculture (Bellwood, 2013). Judging from the mtDNA haplogroup, the Escalon Man is probably a descendant of this group.
We set out to answer the question, ‘Who was the Escalon Man?’ by examining his skeletal remains via radiocarbon dating, mtDNA analysis, and various measurements and observations of his bones and teeth.
Observation and morphological study of the Escalon Man’s temporal bone, humerus, and femur indicated that he was an adult male individual about 170 cm or more in height. He was much taller than the average adult males of the Aeta (150 cm) and Mamanwa (156 cm), indigenous negrito hunter-gatherers in central Luzon and northeastern Mindanao (Omoto, 1989). Detailed observation of the Escalon Man’s bones and teeth indicated that he was likely an early middle-aged farmer who probably engaged in hard, ground-based labor.
The Escalon Man’s teeth are much larger than those of the Aeta people and similar to those of Oceanian and Pacific peoples. In addition, his teeth had relatively low tooth wear scores, indicating that estimations of his age were too young, if he did indeed belong to a hunter-gatherer group. In addition, we were able to examine teeth of a Mamanwan male (Kanazawa et al., 2015), and found that they are relatively large, like those of the Escalon Man. However, they are markedly different in their degree of adult attrition: whereas the Escalon Man’s teeth display only slight attrition, the Mamanwan male’s teeth display considerable attrition, probably due to hard diets common among hunter-gatherers. These findings, together with the Escalon Man’s large stature, agrees with our hypothesis that the Escalon Man was a farmer rather than a hunter-gatherer. Furthermore, since there is no sign of external auditory exostosis, he was possibly not a daily fisherman.
Notably, we found numerous narrow grooves on the surface of the Escalon Man’s right humerus fragment. It is important to determine whether these grooves were made by humans or a non-human agency. Observations with a magnifier and an SEM have thus far failed to decisively determine whether these marks are man-made; however, it also appears unlikely that they were made by animal bites. Therefore, further studies are needed to determine the nature of these marks. Because cut marks are sometimes made on human bones during some kinds of rituals, we propose that ethnographic studies on the Escalon Man’s supposed culture may help in addressing this question.
The radiocarbon date of the Escalon Man was determined by analyzing a bone sample taken from his femur via modern radiocarbon dating methods. We dated the Escalon Man to 2692 ± 39 years BP (uncalibrated), indicating that he lived during the Late Neolithic or the Early Metal Age (Fox, 1970; Jocano, 2001; Bellwood, 2013).
Since Escalon Cave happened to be within Mamanwan territory, it seemed particularly important to determine the Escalon Man’s genetic origins. It is widely known that the Philippines are the crossroads for human migration in Southeast Asia. Apart from the non-sapiens hominin Homo luzonensis discovered in northern Luzon (dated to c. 67000 years BP), several groups of modern humans stemming from out-of-Africa forerunners may have arrived in the Philippines during the late Paleolithic era, roughly 50000–10000 years BP. They are the likely ancestors of the present-day Negritos.
A second large wave of migration to the present-day Philippines took place in the Neolithic era, c. 5000–4000 years ago. This second wave of migrants consisted mostly of agriculturists who originated from southeastern China and Taiwan who rapidly diverged in the Philippine Islands and further dispersed southward to the ISEA and Oceania, ultimately reaching the Pacific Islands and Madagascar. They are believed to have been Austronesian-language speakers (Bellwood, 2013).
Today, it is possible to confidently infer the origins of ethnolinguistic groups of modern humans from mtDNA analyses. Each group is characterized by dominant mtDNA types, called haplogroups. In the present study, DNA was extracted from the Escalon Man’s molar and subjected to mtDNA analyses. After cautious treatments to eliminate external DNA contamination, the Escalon Man’s haplogroup was determined to be E1a1a1a—a sub-haplogroup of E1a1a, which is a known marker of Austronesian-speaking people (Ko et al., 2014). This haplogroup does not characterize indigenous negrito populations, such as the Mamanwa (Delfin et al., 2014). Although the haplogroup E1a1a is reportedly present at a low frequency in Mamanwan DNA samples, it was possibly introduced by intermixing of Mamanwan and adjacent non-Negritos, such as the Manobo.
Three ethnolinguistic groups are known to inhabit the province of Surigao del Norte, Caraga Region (XIII) northeastern Mindanao, where Escalon Cave is situated: the Mamanwa hunter-gatherers, the Manobo tribal group (who originated from Neolithic era, Austronesian-speaking farmers), and the Surigaonon (a non-tribal or urban Filipino group). Assuming that only the Mamanwa and the Manobo populations were living in this area c. 2600–2700 years BP, it is likely that the Escalon Man is an ancestor of the presentday Manobo.
The discovery and analysis of the Escalon Man’s remains begs many questions about the human history of the Philippines. This study has made notable contributions to the archaeological literature of the Philippines due to its integration of physical and biological anthropology, including the use of radiocarbon dating and DNA testing. Such unique elements enabled us to make reasonably accurate inferences about the genetic origins and life of the Escalon Man.
The authors thank the anonymous reviewers for their valuable comments. The authors also wish to thank Dr. Kyriacos Louca for showing us the importance of the finding in Escalon Cave, and would like to express our deep appreciation to Dr. Mary Jane Louise A. Bolunia (Terrestrial Archaeology Division of the National Museum of the Philippines, Manila) for securing two permits to export the Escalon skeletal remains as cultural property from the Philippines to Japan. The authors would like to express deep gratitude to Dr. Yuria Furusawa (Museum of Modern Art of Shiga) and her mother, Mrs. Mari Furusawa, for their assistance in hand-carrying the Escalon specimens to Japan. Professor Kazuhiko Tanaka (Tsurumi University) helped in the transportation of these specimens and provided us with important literature regarding Philippine archaeology. The authors express their deep gratitude to Dr. Jasmin Jiji B. Miranda of Surigao City who made a Mamanwa tooth mold available for dental anthropological study, and to Professor Michael Pietrusewsky (University of Hawaii) and Professor Michiko Into (National Museum of Ethnology, Japan) for providing us with important reference data and useful suggestions. Mr. Eiichiro Noguchi helped K. Omoto in editing the manuscript.
The six sections of this paper were prepared separately and integrated into a single paper by the lead author. The original authorship of each section is as follows: Introduction, K. Omoto, L.E. Bauzon, and F.A. Almeda, Jr.; Morphological study of the Escalon Man’s skeletal remains, H. Baba and K. Sakaue; Morphological study of the Escalon Man’s dental remains, E. Kanazawa; Radiocarbon dating the Escalon Man’s remains, M. Yoneda; Mitochondrial DNA analysis, H. Kanzawa-Kiriyama, K. Shinoda, T. Kakuda and N. Adachi; Overall Summary and Discussion, K. Omoto, L.E. Bauzon, and F.A. Almeda, Jr.
| Sample | Escalon Man | Total | |||||
|---|---|---|---|---|---|---|---|
| Sampling | Upper right first molar tooth | — | |||||
| DNA extraction | Yamanashi University | — | |||||
| Illumina index (Illumina TruSeq LT) | A016 | A014 | A002 | A004 | A007 | A013 | — |
| PEG | − | + | + | + | + | + | — |
| Amount of input DNA for library preparation (μl) | 8 | 8 | 8 | 8 | 8 | 8 | — |
| Type of adapter | Long | Short | Short | Short | Short | Short | — |
| Sequencer | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | — |
| Read length of sequence | 150 bases, PE | 150 bases, PE | 75 bases, PE | 75 bases, PE | 75 bases, PE | 75 bases, PE | — |
| Total reads (R1 + R2) | 2796810 | 2289046 | 3873492 | 3184130 | 4301846 | 4081652 | — |
| Filtering | — | ||||||
| Merging R1 and R2 | 1168430 | 847382 | 1461113 | 1202812 | 1584741 | 1302516 | — |
| Hits to hg19 (%) | 16956 | 14518 | 17194 | 13824 | 17734 | 16673 | — |
| Remove cross contamination | 15592 | 12676 | — | — | — | — | — |
| >3 duplicate reads | 14256 | 11614 | — | — | — | — | — |
| NUMT + mtDNA | — | — | — | — | — | — | 88282 |
| Remapped to rCRS with flag 0 and 16 | — | — | — | — | — | — | 88220 |
| MarkDuplicates | — | — | — | — | — | — | 3303 |
| >=mapq20 | — | — | — | — | — | — | 3303 |
| Depth of mitochondrial DNA | — | — | — | — | — | — | 14.12 |
| (a) Schmutzi mitochondrial DNA contamination estimatesa | |||
|---|---|---|---|
| Average | Lower | Upper | |
| Escalon Man | 13% | 11% | 5% |
| (b) Estimate based on haplogroup specific mutationb | |||||
|---|---|---|---|---|---|
| Sample ID | Escalon Man | ||||
| Haplogroup | Nucleotide position of SNP in rCRS | rCRS | Consensus sequence | Match | Mismatch |
| M9/E | 4491 | G | A | 17 | 2 |
| M9/E | 16362 | T | C | 3 | 0 |
| E | 3027 | T | C | 15 | 0 |
| E | 3705 | G | A | 25 | 2 |
| E | 13626 | C | T | 12 | 1 |
| E | 16390 | G | A | 5 | 0 |
| E1 | 13254 | T | C | 11 | 1 |
| E1 | 14577 | T | C | 6 | 0 |
| E1a | 4248 | T | C | 4 | 1 |
| E1a | 10834 | C | T | 11 | 0 |
| E1a1 | 6620 | T | C | 12 | 1 |
| E1a1ac | 14766 | C | C | 10 | 0 |
| E1a1a1 | 16291 | C | T | 1 | 0 |
| E1a1a1a | 8843 | T | C | 7 | 1 |
| Total | 129 | 9 | |||
| Frequency of contamination (%) [95% CI] | 6.52 [2.40–10.64] | ||||
Nucleotide position 14766 (shown as gray) has same mutation from T to C in both haplogroup E1a1a and HV, which includes rCRS. Threfore, the position was not included in the current contamination estimates.

The depth of mtDNA in the Escalon Man. In most positions, the depth is ≥3, which is the criterion for calling reliable SNPs.

Fragment size distribution of sequence reads mapped to rCRS. Only sequences having mapping quality ≥20 were used. PCR duplicates were removed. The peak of the distribution is 57 bp.

Pattern of postmortem misincorporation. C to T indicates C in reference genome and T in the Escalon Man; and G to A indicates G in reference genome and A in the Escalon Man.