2023 Volume 131 Issue 2 Pages 107-115
2023 Volume 131 Issue 2 Pages 107-115
The dental tissue proportions of human permanent canines are one of only a few sexually dimorphic features that are present in childhood, and therefore offer the opportunity to estimate the sex of immature individuals. This work aims to evaluate for the first time the degree of sexual dimorphism in the three-dimensional (3D) measurements of deciduous canine dental tissues, to assess their potential in sexual assessment. Computed microtomographic techniques have been employed to analyse the maxillary and mandibular deciduous canines of 65 individuals (36 females and 29 males) of known sex and age. The teeth were scanned and the volumes and 3D surface areas of the enamel cup and the dentine–pulp complex were obtained. Our results did not show statistically significant differences in either the absolute or relative dimensions of the enamel and dentine between female and male teeth. We hence conclude that volumes and 3D surface areas of deciduous canine dental tissues do not allow for sex determination, which contrasts with what has been observed in permanent canines by other authors. The differences in the degree of sexual dimorphism in dental tissue proportions between permanent and deciduous canines seem to be due to a decrease in the intersexual variability of the dentine component dimensions. Since the dentine component is a tissue capable of responding to changes in sex hormone concentration levels, our results might indicate that hormones play a more important role in the development of sexual dimorphism in the permanent dentition than previously thought.
Numerous investigations have reported the potential of odontometry for sex estimation of human skeletal remains (e.g. Zilberman and Smith, 2001; Schwartz and Dean, 2005; Saunders et al., 2007; Angadi et al., 2013; Viciano et al., 2013; De Angelis et al., 2015; García-Campos et al., 2018a, 2018b; Sorenti et al., 2019). The fact that teeth form early in an individual’s life has made that the study of dental sexual dimorphism particularly relevant in the case of immature individuals, in which secondary sexual traits are not yet discernible in the rest of the skeletal structure (Viciano et al., 2013). For these reasons, a large number of works have explored the sexual dimorphism present in crown dimensions of both deciduous and permanent teeth (e.g. Stroud et al., 1994; Zilberman and Smith, 2001; Schwartz and Dean, 2005; Harris and Lease, 2005; Cardoso, 2010; Viciano et al., 2013) and, for some years, in the dental tissue proportions of human dentition (Stroud et al., 1994; Harris and Hicks, 1998; Saunders et al., 2007; Smith et al., 2008; Feeney et al., 2010; García-Campos et al., 2018a, 2018b; Sorenti et al., 2019; García-Campos et al., 2020).
The results obtained in these investigations have shown that, in modern human populations, the permanent teeth of male individuals generally have larger crowns than their female counterparts, with the canines being the most dimorphic dental feature (İşcan and Kedici, 2003; Pereira et al., 2010; Ayoub et al., 2014; Peckmann et al., 2016). Furthermore, analysis of the sexual dimorphism present in dental tissue proportions has allowed us to identify a sexually dimorphic pattern in the dimensions of the enamel and dentine of the crown. This pattern is characterized by a larger coronal dentine–pulp complex and relatively thinner enamel in the permanent dentition of males (Stroud et al., 1994; Schwartz and Dean, 2005; Smith et al., 2006; Saunders et al., 2007; Feeney et al., 2010; García-Campos et al., 2018a, 2018b; Sorenti et al., 2019; García-Campos et al., 2020). A similar pattern has been described in other non-human primate species (Hlusko et al., 2004; Guatelli-Steinberg et al., 2009). In 2018, García-Campos and colleagues went a step further and developed a new methodology for sex estimation based on the assessment of dental tissue proportions of permanent canines. The accuracy ratio reached with this methodology was up to 92.3% in modern humans (García-Campos et al., 2018a, 2018b), an accuracy comparable to that of other methods applied to the skull or pelvis (Ferembach, et al., 1980; İşcan and Derrick, 1984; Luo, 1995).
Genetic mechanisms and sex hormones seem to be behind the sexual dimorphism of dental tissues. Dental development is the result of a complex interaction of genetic, epigenetic, and environmental factors that occur throughout an individual’s life. On the one hand, prenatal tooth development seems to be under strong genetic control. In this regard, studies on aneuploidy have demonstrated that genes linked to the X and Y chromosomes influence the development of different dental tissues (e.g. Alvesalo and Portin, 1980; Alvesalo et al., 1991; Alvesalo, 2009; Pentinpuro et al., 2014, 2017). Likewise, studies on human amelogenin loci (AMELX, AMELY) seem to support these results (Fincham et al., 1991, 1999; Salido et al., 1992). On the other hand, sex hormones seem to be involved in the overall size of teeth (Woods et al., 1990; Dempsey et al., 1999; Zilberman and Smith, 2001), the timing of gingival eruption (Zingeser and Phoenix, 1978), and the dimensions of dental tissues (Zilberman and Smith, 2001). Despite the results obtained in all these studies, there are still many unanswered questions about the form and extent of the influence that these factors can have on the regulation of dental tissue dimensions. Deciduous dentition could provide enlightening answers about this issue because, due to its early formation and rapid development, it is under stronger genetic control as well as being less influenced by the environment or variations in sex hormone levels than the permanent dentition (Gingerich, 1974; Kondo and Townsend, 2004).
However, contrary to what happens with permanent canines, the dental tissue proportions of deciduous canines have not been assessed despite their potential relevance. This could be because it is almost unanimously accepted that the degree of sexual dimorphism of the crown is substantially less in deciduous teeth than in permanent teeth (Margetts and Brown, 1978; Axelsson and Kirveskari, 1984; Alvrus, 2000; Kuswandari and Nishino, 2004; Harris and Lease, 2005; Cardoso, 2010). Furthermore, the results obtained in the few odontometric studies on sexual dimorphism in the deciduous dentition have been ambiguous (e.g. Margetts and Brown, 1978; Liversidge and Molleson, 1999; Alvrus, 2000; Zilberman and Smith, 2001; Harris and Lease, 2005; Viciano et al., 2013; Akhlaghi et al., 2014). As a result, an ongoing debate exists about the potential of deciduous tooth traits for sex estimation in subadult individuals (Alvrus, 2000; Harris and Lease, 2005; Cardoso, 2010; López-Lázaro et al., 2018). This highlights the need for new studies that explore, using novel approaches and techniques, large forensic samples of known sex, and standardized measurement protocols, the sexual dimorphism of modern humans’ primary dentition.
This work aims to increase the information available on the dental tissue proportions of present-day populations’ deciduous dentition, according to the following specific objectives: (1) to characterize the sexual dimorphism present in the dimensions (volumes and three-dimensional (3D) surfaces) of the dental tissues of the deciduous canines; and (2) to discuss the possible similarities and differences present in the dental tissue proportions of permanent and deciduous canines.
The sample was selected from the Ratón Pérez Collection (Martínez de Pinillos et al., 2021). This extensive dental collection is housed at the Centro Nacional sobre la Evolución Humana (CENIEH) (Burgos, Spain). The dental pieces included in the Ratón Pérez Collection were collected in six successive campaigns carried out between 2014 and 2019. Although it continues to expand, to date this collection consists of 2977 teeth from voluntary donations and belonging to children of both sexes and whose ages of tooth loss range between 2 and 15 years old. Consent informs were obtained for experimentation with human subjects in compliance with Spanish Law 14/2007 of 3 July 2007 on Biomedical Research (BOE-A-2007-12945), with individuals guaranteed anonymity and confidentiality.
The sample for this study was designed to obtain a similar representation of male and female individuals. These teeth come from individuals from different regions of Spain. Where possible, a maxillary and mandibular tooth from the same individual were included in the sample, but only one antimere per tooth class was selected. We discarded teeth that exhibited any major fractures or lesions that affect hard tissues. Slight fractures were digitally corrected. The criteria described by Molnar (1971) were followed for the evaluation of the degree of wear in each tooth. For the evaluation of the crown measurement, only canines with a degree of wear ≤3 (defined by the absence of an apex on the incisal edge of the crown and the appearance of a dentine point in its place) were considered (Table 1). For the assessment of the basal surface of the crown, canines with a degree of wear of 4 were included (Table 1).
| Wear score | Females | Males | Total | |||
|---|---|---|---|---|---|---|
| Maxillary | Mandibular | Maxillary | Mandibular | |||
| 1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 2 | 0 | 3 | 6 | |
| 3 | 12 | 15 | 14 | 16 | 57 | |
| 4 | 4 | 4 | 1 | 3 | 12 | |
| Total | 17 | 21 | 15 | 22 | 75 | |
In total, in this study 75 deciduous maxillary and mandibular canines were selected belonging to 65 individuals of known sex (36 females and 29 males) and age (between 4 and 12 years old). The sample includes 32 upper and 43 lower canines. Maxillary and mandibular canines were analysed separately.
Microtomographic image acquisition and postprocessingSpecimens were scanned using a Phoenix V|tome|x S microtomographic system (GE Measurement and Control), housed at the CENIEH Microscopy Laboratory, with the following parameters: 100–120 kV voltage, 110–140 μA current and two 0.2 mm copper filters were used (Martínez de Pinillos et al., 2021). The resulting images had a voxel size ranging between 17 and 21 μm.
For the subsequent image processing we used Amira 6.0.0 software (Visage Imaging, Inc., San Diego, CA, USA). First, the teeth were oriented according to their anatomical position (see García-Campos et al., 2018a). Then, a non-local mean filter (Buades et al., 2005) was applied. Finally, the dental tissues (enamel and dentine–pulp complex) were segmented semi-automatically with the Watershed Segmentation tool and by subsequent manual editing.
Dental tissue measurementsThe absolute and relative dimensions of the enamel and dentine were measured following the protocol described by García-Campos et al. (2018a, 2018b) and based on the previous work of Benazzi et al. (2014). To isolate the crown dental tissue volumes and 3D surface areas from those of the root, a smooth curve surface was created which was adapted to the morphology of the cervical line (for more details, see the materials and methods section of García-Campos et al., 2018a, 2018b). As a result, the root was isolated from the crown through a concave and homogeneous curved surface, which was employed to define the variable crown basal surface (BS). Therefore, only the dentine and pulp contained within the enamel cup are considered as part of the coronal dentine–pulp complex.
Hereafter, we measure the 3D dimensions (volumes and surface areas) of the dental crown tissues described by Olejniczak et al. (2008a, 2008b) and based on the indices previously proposed by Martin (1985). The variables included in the study were the coronal volume (Vc, mm3), the volume of the enamel cap (Ve, mm3), the volume of the coronal dentine including the coronal pulp (Vcdp, mm3), the surface area of the enamel–dentine junction (EDJS, mm2), and the outer enamel surface area (OES, mm2). Subsequently, these variables were employed to compute the 3D average enamel thickness index (3DAET = Ve/EDJS, mm); the 3D relative enamel thickness index
Statistical analyses were performed with SPSS software v. 18.0 (SPSS Inc.). The analyses were performed with the maxillary and mandibular canines separately. First, descriptive statistics were calculated and the normal distribution and the equal variance assumption were tested using the Shapiro–Wilk test and Levene’s test, respectively. To see the actual patterns of distribution and to check whether the distribution patterns completely overlap or not, histograms with their respective distribution curves were produced for each of the absolute variables of the male and female samples of maxillary and mandibular canines (see Supplementary Figure 1 and Figure 2). Then, to assess the possible differences between the tissue dimensions of male and female deciduous canines, mean comparison analyses were applied. Student’s t-tests were used when the sample was normally distributed; in the remaining cases a Mann–Whitney U-test was employed. Statistically significant differences were considered at the α = 0.05 level.
Additionally, to evaluate the possible similarities or differences in enamel thickness distribution patterns between female and male subsamples, 3D enamel distribution maps were performed for a selection of eight female canines (four maxillary and four mandibular) and eight male canines (four maxillary and four mandibular) of the analysed sample. The 3D maps are obtained by calculating the distance between two triangulated surfaces (EDJS and OES). For each vertex of one surface, the closest point of the other surface was computed and the results were visualized by spectral colours, with thicker enamel values (greater distance between surfaces) represented in red and thinner ones (smaller distance) in blue.
Finally, to compare the magnitude of sexual dimorphism of the deciduous and permanent dentition, the percentage of sexual dimorphism (PSD) was calculated using the formula of Garn et al. (1967):
To assess the percentage of sexual dimorphism of permanent maxillary and mandibular canines, we employed the data available in García-Campos et al. (2018a, 2018b).
The results of the descriptive statistics are shown in Table 2. This table also shows the results of the Shapiro–Wilk normality test and those of the mean comparative analyses. Figure 1 shows the 3D enamel thickness distribution maps. Supplementary Figure 1 and Figure 2 show the distribution curves of the absolute variables of the male and female samples. Finally, the values of the percentages of sexual dimorphism obtained from deciduous and permanent canines are given in Table 3.
| Maxillary canines | Mandibular canines | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | CS | Females | Males | CS | ||||||||||||||
| N | Mean | SD | S–W | N | Mean | SD | S–W | P-value | N | Mean | SD | S–W | N | Mean | SD | S–W | P-value | ||
| Absolute variables | |||||||||||||||||||
| Ve | 11 | 29.77 | 4.00 | 0.235 | 14 | 34.31 | 6.44 | 0.968 | 0.053 | 15 | 28.68 | 5.58 | 0.083 | 20 | 29.93 | 5.22 | 0.528 | 0.501 | |
| Vcdp | 11 | 66.55 | 7.70 | 0.065 | 14 | 72.22 | 10.90 | 0.599 | 0.158 | 15 | 58.19 | 7.34 | 0.057 | 20 | 61.11 | 9.13 | 0.147 | 0.351 | |
| Vc | 11 | 96.32 | 9.83 | 0.346 | 14 | 106.54 | 16.54 | 0.962 | 0.084 | 15 | 86.86 | 12.66 | 0.028 | 20 | 91.03 | 13.39 | 0.398 | 0.358* | |
| EDJS | 11 | 75.00 | 5.19 | 0.421 | 14 | 79.54 | 6.88 | 0.437 | 0.083 | 15 | 70.66 | 6.36 | 0.066 | 20 | 72.78 | 6.37 | 0.781 | 0.337 | |
| OES | 11 | 95.56 | 6.16 | 0.584 | 14 | 101.84 | 10.27 | 0.556 | 0.087 | 15 | 91.34 | 8.75 | 0.027 | 20 | 93.99 | 8.55 | 0.322 | 0.301* | |
| SB | 15 | 20.07 | 1.94 | 0.253 | 15 | 21.21 | 2.00 | 0.091 | 0.124 | 17 | 19.52 | 1.92 | 0.335 | 23 | 20.10 | 2.38 | 0.776 | 0.418 | |
| Relative variables | |||||||||||||||||||
| 3DAET | 11 | 0.40 | 0.05 | 0.370 | 14 | 0.43 | 0.05 | 0.727 | 0.124 | 15 | 0.40 | 0.05 | 0.770 | 20 | 0.41 | 0.05 | 0.491 | 0.699 | |
| 3DRET | 11 | 9.83 | 1.24 | 0.573 | 14 | 10.31 | 1.01 | 0.438 | 0.301 | 15 | 10.41 | 0.94 | 0.851 | 20 | 10.42 | 1.12 | 0.591 | 0.964 | |
| Vcdp/Vc | 11 | 69.05 | 3.00 | 0.993 | 14 | 67.88 | 2.35 | 0.755 | 0.286 | 15 | 67.16 | 2.00 | 0.491 | 20 | 67.17 | 2.40 | 0.311 | 0.990 | |
| OES/EDJS | 11 | 1.28 | 0.04 | 0.519 | 14 | 1.28 | 0.03 | 0.649 | 0.775 | 15 | 1.29 | 0.21 | 0.394 | 20 | 1.29 | 0.03 | 0.505 | 0.932 | |
| 3DRED | 11 | 7.65 | 0.36 | 0.993 | 14 | 7.79 | 0.28 | 0.757 | 0.285 | 15 | 7.88 | 0.24 | 0.492 | 20 | 7.88 | 0.29 | 0.299 | 0.988 | |
All dimensions are in millimetres. Student’s t-test was used when the variables were normally distributed; in all other cases, the Mann–Whitney U-test was applied (*). Means were determined to be significantly different at the α = 0.05 level. Absolute variables included in the study: Ve, volume of enamel cup; Vcdp, volume of the coronal dentine including the coronal pulp; Vc, coronal volume, EDJS, surface area of the enamel–dentine junction; OES, outer enamel surface area; SB, basal surface area of the crown. Relative variables included in the study: 3DAET, 3D average enamel thickness index; 3DRET, 3D relative enamel thickness index; Vcdp/Vc, relative coronal dentine and pulp complex volume; OES/EDJS, relative outer enamel complexity ratio; 3DRED, ratio of enamel thickness to dentine thickness.
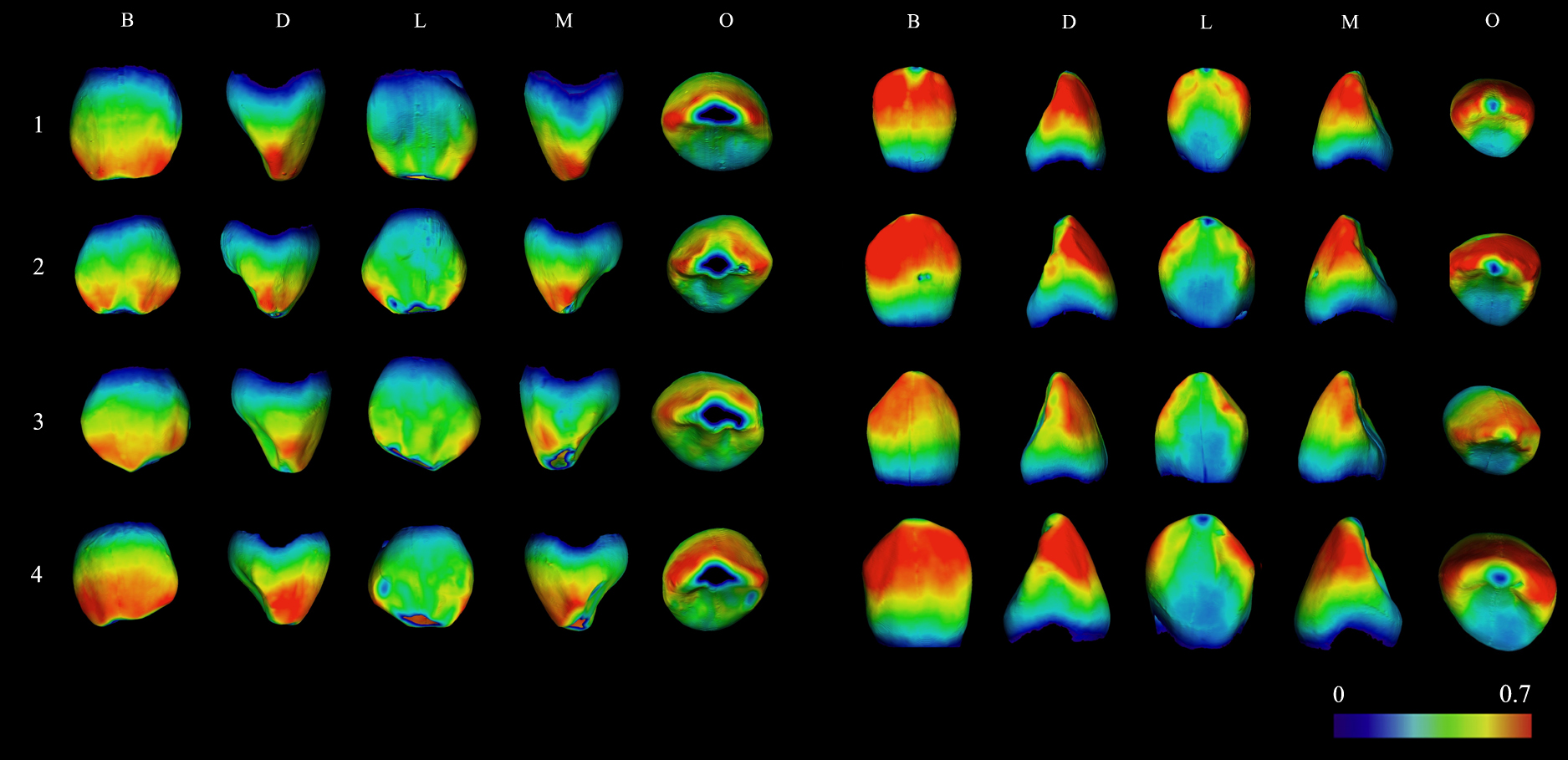
Three-dimensional enamel thickness distribution maps of female (1, 2) and male (3, 4) maxillary (left) and mandibular (right) canines (wear stages 1–3). This figure represents the maps obtained from computing the distance between two triangulated surfaces (enamel–dentine junction surface and outer enamel surface) in which, for each vertex of one surface, the closest point of the other surface is computed. The results are visualized using spectral colours, where the larger distance (thicker enamel) is represented in red and the smaller distance (thinner enamel) in blue. Color scale, 0–0.7 mm. All the teeth represented are right canines; left canines have been mirrored. Views: buccal (B), distal (D), lingual (L), mesial (M) and occlusal (O).
| Degree of sexual dimorphism (%) | ||||||
|---|---|---|---|---|---|---|
| Ve | Vcdp | Vc | EDJS | OES | SB | |
| Maxillary canines | ||||||
| Deciduous | 15.25 | 8.52 | 10.61 | 6.05 | 6.57 | 5.68 |
| Permanent | 17.13 | 38.68 | 28.10 | 23.58 | 17.13 | 32.47 |
| Mandibular canines | ||||||
| Deciduous | 4.36 | 5.02 | 4.80 | 3.00 | 2.90 | 2.97 |
| Permanent | 6.21 | 20.90 | 13.90 | 17.33 | 11.09 | 24.70 |
Ve, volume of enamel cup; Vcdp, volume of the coronal dentine including the coronal pulp; Vc, coronal volume, EDJS, surface area of the enamel–dentine junction; OES, outer enamel surface area; SB, basal surface area of the crown.
The mean values obtained in the descriptive statistical analyses showed that the crown dimensions of the male individuals’ maxillary and mandibular deciduous canines are higher than those of female individuals. This can be seen in the absolute dimensions assessed. Despite this, these differences were not statistically significant in both the absolute and the relative variables measured in this study (P > 0.05, see Table 2 and Supplementary Figure 1 and Figure 2).
The enamel thickness distribution patterns can be appreciated in the 3D colour maps shown in Figure 1. In maxillary and mandibular canines the enamel thickness values were generally higher on the buccal aspect of the tooth, especially in the upper half of the crown. In contrast, the values are lower in the cervical region. This pattern is common in both male and female samples; the latter generally show the slightly highest enamel thickness values, which is reflected by the more intense red colour.
Regarding the differences in the degree of sexual dimorphism between modern human populations’ deciduous and permanent canines, the results obtained in this study showed that the PSD values are lower in the deciduous canines compared to the permanent ones (Table 3). This is particularly notable in those variables related to the coronal dentine–pulp complex dimensions (Vcdp, EDJS, BS), while the differences in the enamel cup are less pronounced.
While the values of the PSD in the deciduous canines are lower, there are notable differences in the values of this percentage for the maxillary and mandibular dentition. For all absolute variables, except Vcdp, the PSD of maxillary deciduous canines is twice that of mandibular canines (Table 3). A similar trend, although to a lesser extent, is observed in the case of the permanent dentition. This points to a higher dimorphism in the maxillary dentition, which is also evident when observing the distribution patterns of these variables (Supplementary Figure 1 and Figure 2). Thus, a high degree of overlap in the distribution curves is observed in the male and female mandibular samples, which, however, tends to dissipate in the maxillary samples, with the male curves shifted towards higher values.
The discovery of a certain degree of sexual dimorphism in some dimensions of the permanent dentition has led several authors to try to assess the degree of sexual dimorphism present in the traits of deciduous teeth, without having obtained conclusive results so far (Kuswandari and Nishino, 2004; Harris and Lease, 2005; Viciano et al., 2013). These studies are few and limited to the assessment of linear measurements from dental crowns (e.g. Liversidge and Molleson, 1999; Zilberman and Smith, 2001; Viciano et al., 2013), whereas the sexual dimorphism of the dental tissue proportions has not been evaluated so far.
For the first time, this study has explored the sexual dimorphism present in the 3D measurements (volumes and surface area) of the crown enamel and dentine–pulp complex of the deciduous dentition. Although no significant differences were found between the deciduous canines of both sexes, our results have allowed us to identify a sexually dimorphic pattern in their dental tissue dimensions. We observed that the male individuals’ deciduous canine crowns (both maxillary and mandibular) were bigger (Vc, SB) than those of female individuals. Likewise, they have higher dentine (Vcdp, EDJS) and enamel (Ve, OES) absolute dimensions. However, taking into account the relative variables, these differences could not be appreciated, which would indicate that the enamel and dentine components might proportionally increase in size in males regarding female canines. Furthermore, the 3D enamel thickness distribution maps show an enamel thickness distribution pattern similar for the deciduous canines of both sexes, with no clear differences.
The degree of sexual dimorphism of the absolute variables shows that the differences are more marked in the maxillary canines than in the mandibular canines, for both the permanent and the deciduous samples. In the case of the latter, this difference is also evident in the distribution curves (Supplementary Figure 1 and Figure 2). Although we cannot rule out that our results may be affected by the limited size of our sample, it is interesting to note that, in the case of the permanent dentition, mandibular canines have been widely used in sex identification (Kondo and Townsend, 2004; Schwartz and Dean, 2005; Saunders et al., 2007; Akhlaghi et al., 2014; Ayoub et al., 2014), while studies focusing on their maxillary counterpart are scarce (Peckmann et al., 2016; García-Campos et al., 2018a). Our results contradict this predominance of mandibular canines in studies on dimorphism. On the other hand, studies of deciduous canines point to a similar pattern to that reported in this paper. Black (1978) also found a higher percentage of sexual dimorphism for maxillary canines than for mandibular canines, in this case referring to the mesiodistal and buccolingual dimensions of the crown. In the case of other teeth, a greater maxillary dimorphism has also been pointed out. López-Lázaro et al. (2018) identified an evident sexual dimorphism in the maxillary and mandibular first deciduous molars, although it was only statistically significant in the maxillary molar. Therefore, further comparative studies on the degree of sexual dimorphism between the maxillary and mandibular dentition could be of interest.
These results contrast with those obtained in the permanent dentition. In 2018, García-Campos and colleagues analysed the degree of sexual dimorphism in dental tissue dimensions of permanent canines using the same methodology applied in this research (García-Campos et al., 2018a, 2018b). These authors observed that all the absolute dimensions of enamel and dentine components were significantly higher in males. Similarly, they noted significant differences in the relative variables assessed. These differences were also found to be sufficiently marked to determine sex with a high degree of accuracy, using both multivariate and univariate equations. This work was based on previous studies by Schwartz and Dean (2005), Saunder et al. (2007), and Feeney et al. (2010) in which cross-sectional crown planes were used to evaluate enamel and dentine dimensions. Although all these studies differ in the methodology employed, they generally agree on the existence of differences in the dimensions of the dental tissues of the permanent dentition of both sexes.
The results obtained here seem to support that the differences in dental tissue dimensions of deciduous canines are not sufficiently marked to propose a new sex determination methodology. Other studies have already noticed this issue. Alvrus (2000) suggested that the application of sex estimation methods based on deciduous dentition could be problematic due to the wide range of variation in tooth size between female and male dentition. Likewise, Cardoso (2010) tested discriminant functions based on the assessment of the crown dimensions of the deciduous dentition proposed by Black (1978), DeVito and Saunders (1990), and Żądzińska et al. (2008). This author noted that these methodologies were not all accurate for sex estimation. However, although our results seem to exclude canines as the key tooth for sex estimation in the primary dentition, this may not be extrapolated to the rest of the deciduous dentition. Previous studies have noted that, on contrary to the permanent dentition, incisors and first and second molars are the most dimorphic teeth in the primary dentition (Yuen et al., 1997; Liversidge and Molleson, 1999; Żądzińska et al., 2008; Viciano et al., 2013). In particular, some authors have referred to the second deciduous molar as the key tooth for sex estimation in the primary dentition (e.g. Liversidge and Molleson, 1999; Harris and Lease, 2005). Therefore, future work assessing the proportions of the dental tissues of other deciduous teeth, in particular the second molars, could yield promising results for the development of new sex estimation methodologies.
Even so, there are justifiable reasons for not giving up the study of deciduous canines for the development of methods in sex estimation of juvenile skeletons. The statistical power required for the development of reliable sex diagnosis methods is significantly conditioned by the reduced sample size of deciduous teeth in osteological collections (Żądzińska et al., 2008). If the difference obtained here is not statistically significant, it may be due to the limited sample size. Also, even if the degree of dimorphism is small, the distribution may still be clearly dichotomous as shown in Supplementary Figure 1 and Figure 2. The maxillary canines of males are overall larger than those of females by the measurement of absolute variables (Ve, EDJS, OES; Table 2). Thus, it is too early to conclude categorically that the intersex difference is not sufficiently marked in deciduous canines for sex determination, or, at least, for sex estimation. Even though our results are not sufficient to justify their usage as a standalone method to differentiate between sexes in subadult individuals, the new approach presented here maintains the potential importance for larger sample size, or inclusion of other populations and new methods (Viciano et al., 2021; Sasaki et al., 2021).
On a different note, it should also be highlighted that the results obtained here from assessment of deciduous canines are very different to those obtained from the analysis of permanent dentition, in general, and of permanent canines, in particular (e.g. İşcan and Kedici, 2003; Acharya and Mainali, 2008; Hassett, 2011; Zorba et al., 2013; Peckmann et al., 2016; García-Campos et al., 2018a, 2018b). The results of permanent dentition studies showed that there are several dental variables that can be used to develop sample-specific methods for sex determination and that the correct allocation accuracies that they achieve are comparable to other metric methods commonly applied to other skeletal elements (Acharya and Mainali, 2008; Hassett, 2011; Zorba et al., 2013; García-Campos et al., 2018a, 2018b). In particular, the usefulness in sex estimation studies of the multivariate methods based on linear dimensions of the canines has been deeply explored, yielding accuracies ranging from 67% to 88.2% (e.g. İşcan and Kedici, 2003; Zorba et al., 2013; Peckmann et al., 2016). In 2018, García-Campos and colleagues were a step further and explored the potential of dental tissue dimensions of permanent canines for sex estimation. The results obtained in both studies (on maxillary and mandibular permanent canines) enabled the development of a discriminant function model that achieves a correct assignation rate of 92.30% (García-Campos et al., 2018a, 2018b). As can be observed, these results are far from those obtained in the present study from the study of deciduous canines.
To try to explain these differences, we compared the results obtained here from deciduous canines with those from permanent canines reported by García-Campos et al. (2018a, 2018b). To do that, we employed the PDS (Garn et al., 1967). We discovered that the differences in the degree of sexual dimorphism between the dental tissue proportions of permanent and deciduous canines seems to be due to a decrease in the differences in the dentine component between male and female dental features. We also realised that the values of PSD decreased notably in deciduous canines in all those variables related to the dimensions of the dentine–pulp complex (Vcdp, EDJS, BS). In contrast, the values of the enamel component (Ve, OES), although always lower in deciduous canines, are not affected to the same extent as those of the dentine component. These findings agree with previous work’s conclusions, in which it was proposed that the greater dentine–pulp complex in males is behind the differences in tooth size between males and females, whereas the enamel component does not contribute significantly to sexual dimorphism in tooth size (Alvesalo et al., 1991; Stroud et al., 1994; Schwartz and Dean, 2005; Saunders et al., 2007; García-Campos et al., 2018a, 2018b).
The results of this study may help to understand the regulatory mechanisms underlying the sexual dimorphism present in the tissue dimensions of the permanent dentition. Unlike enamel, dentine is a living tissue capable of responding to different biological stimuli and signals even after tooth formation is complete. Zilberman and Smith (2001) studied the hypothesis that odontoblast activity is influenced by sexual hormones, and is expressed in differences in dentine thickness that change with age. They concluded that the origin of differences in dental tissue proportions is probably due to the different rates of dentine deposition taking place during the lives of males and females. It is relevant to note that the differences in dental dimorphism in the dimensions of the dental tissues between deciduous and permanent canines observed in this study are precisely in the dentine component. Therefore, although it is not the subject of this work, to better understand the results obtained in this study, it would be interesting to describe how the formation times of different tooth classes correlate with the variations in sex hormone concentrations that occur throughout an individual’s life.
Calcification in permanent teeth starts several months after birth, except for the first permanent molars (which start at birth), reaching their definitive size and shape between 2 months and 8 years of age, with the exception, once again, of the third molars (Mendoza and Solano, 2005). This overlaps with an increase in plasma concentrations of sex steroid hormones described by Quigley (2002). This author observed that infants experience a postnatal increase in gonadotropin and sex steroids during the first 6 months of life, reaching pubertal levels. This increase, moreover, occurs with differences in timing, character, and intensity in individuals of each sex (Quigley, 2002). In contrast, deciduous teeth, whose calcification begins in utero between 13 and 18 weeks after fertilization (Lunt and Law, 1974; Sunderland et al., 1987), are not affected by the increase in gonadotropin and steroid hormones observed by Quigley (2002). In the particular case of deciduous canines, their hard-tissue calcification begins between the 15th and the 18th week after fertilization and finishes around the 9th month after birth, only followed by the second molar which completes the mineralization of its crown around 11 months (Lunt and Law, 1974). Therefore, these teeth would presumably be more influenced than the other tooth classes by the hormonal changes described by Quigley (2002). However, at six months of age, the formation of the deciduous canine crown would be already well advanced, so that the influence of this event is limited, while the crown of the permanent canines would have already started to form at that time (Mendoza and Solano, 2005). Additionally, at later stages, the dentine dimensions of the permanent dentition may be altered by other changes in postnatal sex hormone levels (Zilberman and Smith, 2001), which would affect the proportions in which the different dental tissues are expressed. Gingerich (1974) hypothesized that the dental classes that form during early childhood are less dimorphic than those that form later in life. The hypothesis proposed by Gingerich (1974) was based on the fact that postnatal hormones increase throughout an individual’s life, peaking in adolescence. Hence, the differences in the correlation between the dental formation times and variations in postnatal sex hormone concentrations could be behind the results obtained in this study. Future studies may help to clarify to what extent the sex hormones affect dental tissue dimensions, and therefore either support or refute the hypotheses proposed in this study.
This study has been supported by the Dirección General de Investigación of the Spanish Ministerio de Economía y Competitividad (MINECO/FEDER) grant number PGC2018-093925-B-C31, and The Leakey Foundation through the personal support of G. Getty (2013) and D. Crook (2014–2021) to M. M.-T. C.G.-C. is the beneficiary of a postdoctoral research grant Cajaviva Fundación Caja Rural Burgos-Atapuerca Foundation. We thank the ‘la Caixa’ Foundation and the Caja de Burgos Foundation which fund the creation of the Ratón Pérez collection. The micro-CT images were obtained in the Microscopy Laboratory of CENIEH-ICTS (Spain) in collaboration with CENIEH staff. In particular, we are indebted to Ana Pantoja Pérez for their dedicated work. Finally, we would like to acknowledge Dr Laura Martín-Francés for her collaboration in the creation of this collection.