2019 Volume 2 Issue 6 Pages 106-112
2019 Volume 2 Issue 6 Pages 106-112
Versican is a large aggregating chondroitin sulfate proteoglycan that accumulates in vascular wall during atherosclerosis. This proteoglycan is particularly synthesized by arterial smooth muscle cells and contributes to atherosclerosis progression by enhancing the retention of low density lipoproteins and inducing the proliferation and migration of the cells in atherosclerotic plaques. There is a strong interrelationship between atherosclerosis and thrombosis, suggesting that thrombin—a key coagulation factor—may stimulate versican synthesis in arterial smooth muscle cells. To determine the regulation of proteoglycan synthesis by thrombin receptor agonist peptide (SFLLRN), cultured human coronary smooth muscle cells were stimulated by the peptide, and proteoglycans synthesized by the cells were characterized by biochemical techniques. The experiments indicate that SFLLRN enhances the synthesis of versican V0 variant without affecting the length and disaccharide composition of its chondroitin sulfate chains under high cell density condition. This suggests that the procoagulant state of blood may accelerate atherosclerosis progression through a high accumulation of versican V0 variant derived from arterial smooth muscle cells after the cell density becomes higher in atherosclerotic plaques.
Proteoglycans (PGs) are macromolecules composed of a core protein and one or more glycosaminoglycan (GAG) side chains as a common feature. Versican is a chondroitin sulfate PG (CSPG) present in the extracellular matrix of vascular wall.1) Although PGs generally accumulate in vascular diseases such as atherosclerosis, the type of PGs that accumulate in the expanded intima of the vascular wall varies depending on the stage of the vascular lesion. Versican as well as biglycan and decorin—small leucine-rich dermatan sulfate PGs (DSPGs)—excessively accumulate in both intermediate and advanced atherosclerotic plaques, whereas the expression of perlecan—a basement membrane heparan sulfate PG (HSPG)—is low in intermediate lesions but high in advanced lesion in areas bordering the plaque core.2) Among the PGs that accumulate during atherosclerosis, versican plays a key role in the progression of atherosclerosis and restenosis3) by interacting with growth factors/cytokines, enzymes, lipoproteins, and other extracellular matrix components.4–8) It has been reported that the source of versican in the blood vessel wall is vascular smooth muscle cells,9) and the synthesis of this CSPG is induced by various stimuli, including platelet-derived growth factor (PDGF),10) transforming growth factor-β (TGF-β),10) mechanical strain,11) oxidized low density lipoproteins (Ox-LDL),12) and angiotensin II.13)
Four different isoforms of versican are generated by alternative splicing of mRNA from a single gene.14,15) The splicing occurs in 2 large exons, namely, exons 7 and 8, which encode the GAG attachment sites, thereby forming 4 versican variants, i.e., V0, V1, V2, and V3.16) These variants differ in the size of the core proteins and number of attached chondroitin sulfate chains.16,17) It has been shown that vascular smooth muscle cells synthesize V0, V1, and V3 but do not express V2.18) The core protein of V3 lacks the ability to bind chondroitin sulfate chains but influences the adhesion, migration, proliferation, and elastic fiber assembly of vascular smooth muscle cells.19–21) On the other hand, the V0 and V1 variants interact with hyaluronan, a long-chain GAG in the extracellular matrix; the versican-hyaluronan aggregates are required for the proliferation and migration of vascular smooth muscle cells.22) In addition, chondroitin sulfate chains of versican V0 and V1 variants have native low density lipoprotein (LDL) binding properties and form insoluble complexes with LDL.23–25) The formation of versican-LDL complexes accelerates not only oxidation of LDL but also uptake of LDL by macrophages.26) Thus, versican V0 variant contributes to atherosclerosis progression by stimulating arterial smooth muscle cells and enhancing LDL accumulation in the plaques.
Although there is a strong interrelationship between atherosclerosis and thrombosis, it is unclear whether the procoagulant state of blood influences the synthesis of PGs in arterial smooth muscle cells during atherosclerosis. Thrombin is the key enzyme in the blood coagulation-fibrinolytic system. This protease not only converts fibrinogen to fibrin but also stimulates arterial smooth muscle cells mostly via activation of protease-activated receptor-1 (PAR-1). Expression of PAR-1 is high in atherosclerotic arteries,27) suggesting that the regulation of arterial smooth muscle cell functions mediated by PAR-1 plays an important role in atherosclerosis progression. In fact, activation of PAR-1 stimulates the proliferation28) and synthesis of procollagen29) in vascular smooth muscle cells.
We have demonstrated that thrombin induces the synthesis of perlecan in cultured human coronary smooth muscle cells.30) However, it is unclear whether the regulation of PG synthesis in arterial smooth muscle cells is mediated by thrombin receptor. In addition, it is possible that the receptor mediates the regulation of versican and biglycan/decorin synthesis as well as HSPG synthesis in vascular smooth muscle cells. In the present study, in order to examine this possibility, cultured human coronary smooth muscle cells were stimulated by synthetic thrombin receptor agonist peptides,31) and synthesized PGs were characterized. The results suggest that one of the peptides, SFLLRN, enhances the synthesis of versican V0 variant without affecting the length and disaccharide composition of its chondroitin sulfate chains in the cells.
Human coronary artery smooth muscle cells were cultured in HuMedia SG-2 in 100-mm dishes in a humid atmosphere of 5% CO2 in air until confluence. The cells were transferred into 24-well plates or 100-mm dishes at 1 × 104 cells/cm2 and cultured for 24 h (“sparse cultures”) or until confluence (“dense cultures”). After washing the cells with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% BSA, the medium was replaced to test media made with fresh DMEM supplemented with 1% BSA. After then, the experiments were performed as described below.
Incorporation of [35S]Sulfate into GAGsThe incorporation of [35S]sulfate into GAGs accumulated in the cell layer and the conditioned medium of cultured human coronary artery smooth muscle cells was determined by the CPC precipitation method32) as described previously.30) Briefly, dense and sparse cultures of the cells were prepared and incubated at 37 °C for 8, 24, or 48 h with a synthetic peptide (SFLLRN, SLIGKV, NRLLFS, or VKGILS) at 20, 50, 100, or 200 µM in DMEM supplemented with 1% BSA with [35S]sulfate (1 MBq/mL). The radiolabeled PGs were extracted in the presence of 8 M urea; the PGs were precipitated by 1% cetylpyridinium chloride and the incorporated radioactivity was measured by liquid scintillation counting.
Characterization of PGsThe [35S]sulfate-labeled PGs extracted from the cell layer and the conditioned medium of dense cultures of human coronary smooth muscle cells treated with the synthetic peptide SFLLRN (100 µM) for 48 h in DMEM supplemented with 1% BSA in the presence of [35S]sulfate (3 MBq/mL) were characterized by Sepharose CL-2B and DEAE-Sephacel columns before and after digestion of heparan sulfate or chondroitin/dermatan sulfate chains with heparinase II/III or chondroitin ABC lyase, respectively, as described previously.30) The GAG chain size was estimated according to a previously published curve of log Mr versus Kav in Sepharose CL-6B chromatography before and after digestion of heparan sulfate or chondroitin/dermatan sulfate chains or core proteins with heparinase II/III or chondroitin ABC lyase or papain, respectively.33)
Analysis of PG Core ProteinsCore proteins of dense human coronary smooth muscle cells were metabolically labeled with Tran35S-label reagent (3 MBq/mL) in the presence of the synthetic peptide SFLLRN (100 µM) for 48 h in DMEM supplemented with 1% BSA and separated by SDS-polyacrylamide gel electrophoresis on an acrylamide 4–12% gradient slab gel with a 3% stacking gel according to the procedure of Laemmli,34) after digestion of heparan sulfate or chondroitin/dermatan sulfate chains with heparinase II/III or with chondroitin ABC lyase, respectively. Western blot analysis was performed as described previously30) using rabbit antiserum against the versican core protein that was kindly provided by Dr. Richard LeBaron (University of Texas, TX, USA).
Real Time RT-PCRIn another experiment, vascular smooth muscle cells were treated with SFLLRN (100 µM) for 12, 24, 36, or 48 h, and cDNA was synthesized as described previously.35) Real-time RT-PCR was performed in 20 µL reaction using Gene Ace SYBR qPCR Mix α with 2.5 ng cDNA and 0.2 µM primers on a CFX Connect Real-Time PCR Detection System. The levels of versican variants (V0 and V1) and β2-microglobulin (B2M) mRNAs were quantified by the relative standard curve method. The fold change of the intensity value of these variants were normalized by that of B2M. The sequences of human gene-specific forward and reverse primers were as follows: V0, 5’-CAGCCCCCAGCAAGCAC-3’ (forward) and 5’-ATCTGTTTCTTCACTACAAGGTTCATC-3’ (reverse), V1, 5’-GCGATTACGGGTGGCTG-3’ (forward) and 5’-ATCTGTTTCTTCACTACAAGGTTCA-3’ (reverse), B2M, 5’-TCCAAAGATTCAGGTTTACTCACG-3’ (forward) and 5’-GTTCACACGGCAGGCATACTC-3’ (reverse).
Analysis of Disaccharide Composition of Chondroitin SulfateFluorophore-assisted carbohydrate electrophoretic analysis was used to analyze the dissacharide composition of chondroitin sulfate chains of PGs that accumulated in the cell layer of dense cultures of human coronary smooth muscle cells treated with SFLLRN (100 µM) for 48 h, as described previously,30) using gal 6S as the standard.
Statistical AnalysisData were analyzed for statistical significance by Student’s t-test or analysis of variance and Bonferroni’s multiple t-test, whenever possible. P values of less than 0.05 were considered to indicate statistically significant differences.
Figure 1A shows the accumulation of [35S]sulfate-labeled PGs in the cell layer and conditioned medium of human coronary smooth muscle cells after treatment with the synthetic peptide SFLLRN. The [35S]sulfate-labeled PG accumulation in the cell layer significantly increased after a 48-h treatment with 10 µM and higher concentration of the peptide, with no changes in the accumulation in the conditioned medium (Fig. 1A). Since SFLLRN activates PAR-2 as well as PAR-1,36) the effect of SLIGKV, a peptide that specifically activates PAR-2,36) was investigated (Fig. 1B). In this experiment, sparse cultures were also tested to determine whether the response of arterial smooth muscle cells to SFLLRN is cell density dependent. In dense cultures, the increased accumulation of [35S]sulfate-labeled PGs in the cell layer was observed after treatment with SFLLRN; however, the PAR-2-activating peptide SLIGKV and retropeptides of SFLLRN and SLIGKV—NRLLFS and VKGILS, respectively—failed to increase PG accumulation. On the other hand, in sparse cultures, all the peptides failed to affect PG accumulation in the cell layer and conditioned medium. These results suggest that activation of PAR-1 results in an increase in PG accumulation in the cell layer of cultured human coronary smooth muscle cells under high cell density condition.

[35S]Sulfate Incorporation into the GAGs that Accumulated in the Cell Layer (Left Panels) and Conditioned Medium (Right Panels)
[A] Dense cultures of the cells were incubated with SFLLRN (10, 30, 50, 100, and 200 µM) at 37 °C for 48 h in the presence of [35S]sulfate. [B] Dense and sparse cultures of the cells were incubated with SFLLRN, SLIGKV, NRLLFS, and VKGILS (100 µM each) at 37 °C for 48 h in the presence of [35S]sulfate. Values are represented as means ± S.E. of 4 samples. ** P < 0.01 vs. corresponding control. ++ P < 0.01 vs. corresponding retro-peptide.
In order to characterize the cell layer-associated PGs whose accumulation is increased by SFLLRN, [35S]sulfate-labeled PGs obtained from the cell layer were separated by Sepharose CL-2B molecular sieve chromatography on the basis of their hydrodynamic sizes (Fig. 2A). The PGs were eluted at Kav of 0.35 (the high Mr subclass) and 0.75 (the low Mr subclass); SFLLRN selectively increased the radioactivity of the high Mr subclass without affecting their hydrodynamic size.

Characterization of PGs and GAG Chains Extracted from Dense Vascular Smooth Muscle Cells after Treatment with SFLLRN in the Presence of [35S]Sulfate
[A] Sepharose CL-2B molecular sieve chromatography of [35S]sulfate-labeled extracts from the cell layer (upper panels) and medium (lower panels) of dense vascular smooth muscle cells. The fractions within the range of horizontal bars are pooled as high Mr subclass. [B] DEAE-Sephacel ion-exchange chromatography of the high Mr subclass. The fractions within the range of horizontal bars are pooled as high charged molecules. [C] Sepharose CL-6B column chromatography of high charged molecules. The fraction was analyzed before and after digestion with heparinase II/III, chondroitinase ABC, or papain.
PGs bound to the high Mr subclass were further purified by DEAE-Sephacel ion-exchange chromatography on the basis of differences in their charge densities (Fig. 2B). The subclass contained 2 populations: the major population eluted by approximately 0.5 M NaCl and the minor population eluted by approximately 0.4 M NaCl. It was observed that SFLLRN markedly increased the [35S]sulfate radioactivity of both populations.
The composition and size of GAG chains of the major population were analyzed by Sepharose CL-6B chromatography (Fig. 2C). The population eluted at the void volume was sensitive to digestion with either chondroitinase ABC or papain but resistant to digestion with heparinase II/III; this suggests that PGs whose accumulation was increased by SFLLRN in the cell layer were CS/DSPGs. The size of the chondroitin/dermatan sulfate chains was Mr~52,000 before and after treatment with SFLLRN, indicating that the peptide did not affect GAG elongation.
SFLLRN Induces the Synthesis of Versican V0 Variant in Human Coronary Smooth Muscle CellsIn order to identify the CS/DSPG core proteins whose accumulation in the cell layer was increased by SFLLRN, PGs were metabolically labeled with 35S-labeled amino acids and separated by SDS-polyacrylamide gel electrophoresis (Fig. 3A). The large CS/DSPG core proteins whose accumulation was increased by SFLLRN were observed as 2 bands with a molecular mass of approximately 510 and 430 kDa. Although both bands reacted with an anti-versican antibody, SFLLRN markedly increased the accumulation of the 510-kDa immunostained versican core protein (Fig. 3A side panel), suggesting that the peptide increases the accumulation of the large versican variant in the cell layer. Since V2 variant is absent18) and V3 variant lacks GAG-binding ability,19) the versican core proteins showing bands at 510 and 430 kDa are postulated to be V0 and V1 variants, respectively. Therefore, it is suggested that SFLLRN specifically increases the accumulation of versican V0 variant in arterial smooth muscle cell layers.
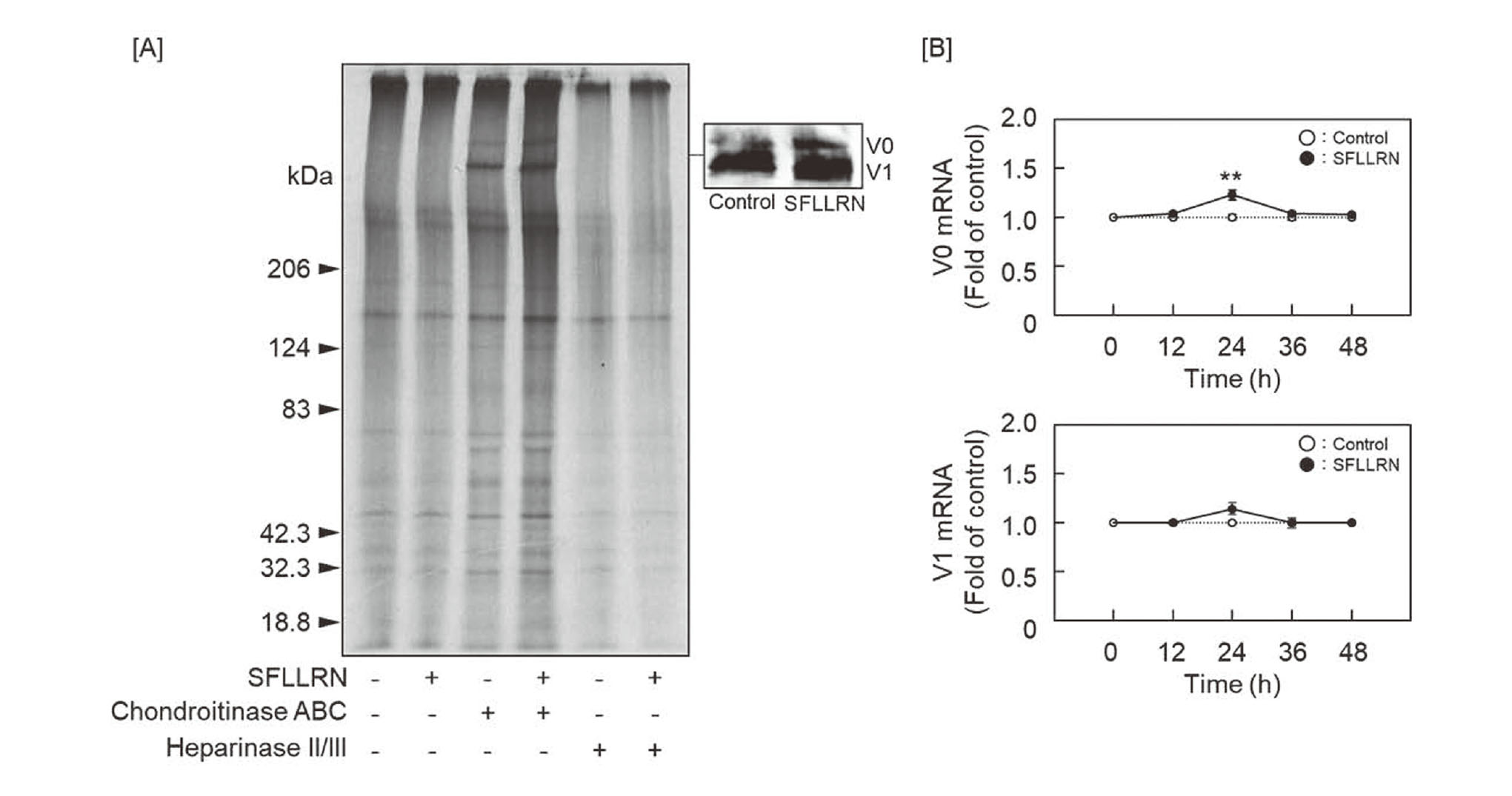
The Expression of Versican Core Protein and mRNA in Dense Vascular Smooth Muscle Cells after Treatment with SFLLRN
[A] 35S-amino acids labeled proteins analyzed by autoradiography (left panel). Versican V0 and V1 core proteins were detected by western blotting after digestion with chondroitinase ABC (right panel). [B] Versican V0 and V1 mRNAs expression. Values are represented as means ± S.E. of 4 samples. *P < 0.05; **P < 0.01 vs. corresponding control.
Since it is suggested that SFLLRN may induce the synthesis of versican V0 variant, the expression level of mRNA for V0 and V1 variants was analyzed by real time RT-PCR (Fig. 3B). The level of V0 mRNA was increased at 24 h after treatment with SFLLRN, while the peptide failed to exhibit such an effect on V1 mRNA.
SFLLRN Does Not Affect the Disaccharide Composition of Chondroitin Sulfate Chains in the Human Coronary Smooth Muscle Cell LayerIn order to determine whether SFLLRN affects the disaccharide composition of chondroitin sulfate chains of versican V0 variant, the disaccharide units generated by digestion with chondroitin ACII lyase were analyzed by fluorophore-assisted carbohydrate electrophoresis (Fig. 4A). The following disaccharide units were detected: unsaturated glucoronic acid-N-acetylgalactosamine [GlcA-GalNAc(0S)], GlcA-GalNAc-6-sulfate [GlcA-GalNAc(6S)], GlcA-GalNAc-4-sulfate [GlcA-GalNAc(4S)], and GlcA-GalNAc-4,6-sulfate [GlcA-GalNAc(4S,6S)]. SFLLRN did not affect the percentage of the disaccharide units (Fig. 4B). Since the main source of chondroitin sulfate disaccharide units in the cell layer are postulated to be versican V0 and V1 variants, it is suggested that the microstructure of chondroitin sulfate chains of versican is not affected by SFLLRN.

Fluorophore-Assisted Carbohydrate Electrophoresis Analysis of Chondroitin Sulfate Disaccharides in the PGs Extracted from the Cell Layer of Dense Vascular Smooth Muscle Cells after Treatment with SFLLRN
[A] A representative gel image. [B] The percentages of the disaccharide units in chondroitin sulfate chains. Values represent the means of 4 different experiments.
Blood is generally in a procoagulant state under atherosclerotic conditions, suggesting a hypothesis that cellular events mediated by PAR-1, which is activated by thrombin, contribute to atherosclerosis progression. In fact, activation of PAR-1 by thrombin results in the stimulation of vascular smooth muscle cell proliferation in vitro,28) suggesting that the mechanism of vascular smooth muscle cell hyperplasia in atherosclerotic plaques involves the stimulation of proliferation mediated by PAR-1. On the other hand, excess accumulation of extracellular matrix, which is composed of collagen, elastin, PGs, fibronectin, laminin, and other minor components, in the plaques37,38) is an important pathological change in atherosclerosis; however, among protease-activated receptors, PAR-1 activation only induces migration and proliferation of vascular smooth muscle cells, and PAR-1 inhibitor suppresses the effects induced by PAR-1 agonist peptide.39–41) Moreover, PAR-1 upregulates the synthesis of procollagen, hyaluronan synthase 2, MMP 7, and TNF-α in vascular smooth muscle cells.28,39,40) In the present study, it was shown that a thrombin receptor agonist peptide, SFLLRN, regulates the PG synthesis in cultured human coronary artery smooth muscle cells. Specifically, the peptide induces the synthesis of CSPG versican V0 variant without marked changes in the length and disaccharide composition of chondroitin sulfate chains under high cell density condition. Previously, we showed that thrombin induces the synthesis of large HSPG, perlecan, in human coronary artery smooth muscle cells.30) Considering this report, perlecan is present around Kav 0.4-0.6 and eluted by approximately 0.4 M NaCl (Fig. 2A and B) in the DEAE-Sephacel chromatography. Since the radioactivity of the fraction was increased by SFLLRN treatment (Fig. 2A), it is suggested that induction of perlecan synthesis by thrombin is mediated by PAR-1. Therefore, the present data suggest that PAR-1 mediates the synthesis of versican V0 variant as well as perlecan in vascular smooth muscle cells at a high cell density.
The core protein of versican variants are constructed with common N- and C-terminal domains with or without of GAG-attachment domain(s). The exons which coding GAG-attachment domains can undergo differential splicing,14,15) resulting in different numbers of chondroitin sulfate chains attached to the core protein. Versican V0 variant contains two GAG attachment regions, αGAG domain and βGAG domain; V1 contains the βGAG domain; V2, which is not expressed by vascular smooth muscle cells,18) contains the αGAG domain; and V3 contains no GAG domain.19–21) Since versican interacts with LDL via chondroitin sulfate chains,23–25) the contribution of versican to atherosclerosis progression would depend on the number and microstructure of GAG chains. Thus, induction of V0 synthesis by activation of PAR-1 suggests that the procoagulant state of blood may result in an increased retention of LDL in the extracellular matrix of arterial smooth muscle cells. On the other hand, versican (V0 and V1 variants) forms “versican-hyaluronan rich matrix” around vascular smooth muscle cells22) and may contribute to hyperplasia of the cells in atherosclerotic vascular wall.
Based on the present results, we suggest the following hypothesis regarding the cellular events during atherosclerosis: thrombin is generated by functional damage of endothelial cells42) and activates PAR-1, which is upregulated in arterial smooth muscle cells of atherosclerotic vascular wall,27) leading to induction of the synthesis of versican V0 variant as well as perlecan,28) and biglycan.43) The increased V0 variant particularly enhances the retention of LDL, followed by generation of oxidized LDL that is toxic to vascular endothelial cells.44) Furthermore, the damaged endothelial cells would reduce the anticoagulant properties of the cells. Therefore, induction of versican V0 synthesis mediated by PAR-1 would serve as a positive feedback loop that accelerates atherosclerosis progression through interaction between vascular cells and abnormal LDL metabolism such as excess LDL accumulation and oxidation. On the other hand, since neointimal formation is reduced in PAR-1 knockout mice,45) activation of PAR-1 may contribute to arterial smooth muscle cell hyperplasia in atherosclerotic vascular wall through the formation of versican (V0)-hyaluronan rich matrix around the cells that is indispensable for their migration and proliferation.22) Promotion of vascular smooth muscle cell proliferation by activation of PAR-1 and anti-restenosis effect against balloon angioplasty by PAR-1 inhibitor28,39,40) supports our hypothesis.
The induction of V0 synthesis by SFLLRN in arterial smooth muscle cells was observed only under high cell density condition. Induction of perlecan synthesis by thrombin also occurs at a high cell density.30) The response of the cells to growth factors/cytokines is often cell density dependent. For example, TGF-β inhibits the growth at a low cell density but promotes it at a high cell density46) and TNF-α inhibits the growth47) and collagen synthesis48,49) in arterial smooth muscle cells only under high cell density condition. Although the mechanism of versican V0 induction by SFLLRN is unclear, it may be strictly regulated by such a cell density dependent signal transduction and contribute to the diverse characteristics of morphology in atherosclerosis. Transactivation of PAR-1 and TGF-β receptor50,51) may mediate the versican V0 induction by SFLLRN, although involvement of other intracellular signaling cannot be excluded. Specifically, the mechanisms for excess accumulation of versican V0/V1 and perlecan in advanced atherosclerotic plaques,2) where arterial smooth muscle cells are generally densely present, may include the regulation of PG synthesis mediated by PAR-1 in the cells. The present data support the hypothesis that the procoagulant state of blood that results from decreased anticoagulant activity of vascular endothelial cells contributes to the progression of atherosclerosis progression through the regulation of arterial smooth muscle cell PG synthesis.
This work was supported by JSPS KAKENHI Grant Numbers JP 19K19418 (to T.H.), JP 18K06638 (to C.Y.), and JP 19K07089 (to T.K.).
Conflict of interestThe authors declare no conflict of interest.