2020 Volume 3 Issue 2 Pages 70-75
2020 Volume 3 Issue 2 Pages 70-75
Myeloid-derived suppressor cells (MDSCs), which are derived from immature bone marrow cell (BMC) populations that proliferate in the tumor microenvironment, suppress T cell immune responses. Transient receptor potential vanilloid (TRPV) 4, which is a Ca2+ channel, is involved in tumor growth, but the role of TRP channels in MDSC differentiation and function remains unclear. Here, we first investigated the involvement of TRP channels in the differentiation of MDSCs. The selective TRPV4 channel antagonist RN-1734 increased the population of MDSCs (CD11b+Gr-1+) at Day 3, while the TRPV4 agonist GSK1016790A decreased it, suggesting that stimulation of TRPV4 suppresses the differentiation of BMCs to MDSCs. GSK1016790A also increased the production of nitric oxide and reactive oxygen species, but suppressed the expression of Arg-1 mRNA, which encodes arginase-1, in MDSCs. Furthermore, GSK1016790A decreased the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in MDSCs, thereby attenuating STAT3 signaling. Our results suggest that TRPV4 plays a role in regulating both the differentiation and function of MDSCs, and therefore could be a promising target for cancer immunotherapy.
The tumor microenvironment contains many immunosuppressive factors and cells,1) including myeloid-derived suppressor cells (MDSCs).2) MDSCs, which co-express CD11b and Gr-1 antigens, are generated by the differentiation of immature bone marrow cells (BMCs) in response to stimulation by various factors (interleukin (IL)-4, IL-6, granulocyte-macrophage colony-stimulating factor, etc.) in the tumor tissue.2,3) MDSCs are heterogeneous, and can be roughly divided into granulocytic MDSCs (G-MDSCs), which are also known as polymorphonuclear MDSCs (PMN-MDSCs), and monocytic MDSCs (M-MDSCs).2,3) The main mechanism of immunosuppressive action of MDSCs is suppression of T cell function and proliferation via expression of arginase-1 (Arg-1), production of nitric oxide (NO) by inducible nitric oxide synthase (iNOS), and production of reactive oxygen species (ROS).2,4) MDSCs also promote recruitment of regulatory T cells (Tregs) by production of TGF-β, recruitment of tumor-invasive macrophages by chemokine production, and tumor metastasis by production of angiogenesis promoting factor (VEGF).5–7) Thus, MDSCs inhibit antitumor immunity in the tumor microenvironment and promote tumor growth; however, the mechanisms of differentiation of BMCs to MDSCs and the acquisition of immunosuppressive ability by MDSCs remain unclear.
Transient receptor potential vanilloid (TRPV) 4 is one of the transient receptor potential (TRP) superfamily, which, in mice, consists of 28 channels in 6 subfamilies: TRPC (canonical) 1-7, TRPV (vanilloid) 1-6, TRPM (melastatin) 1-8, TRPA (ankyrin) 1, TRPP (polycystin) 1-3, and TRPML (mucolipin) 1-3 (in humans the TPRC2 gene is considered to be a pseudogene, while a member of a seventh subfamily, TRPN (NOMPC) 1, is expressed in some other species).8) TRP channels are directly and indirectly activated by various physiological substances and thus act as sensors in cells.9) They interact with various proteins and play a role in intracellular signal transduction via Ca ion influx and depolarization.10) Among them, the TRPV4 channel is involved in tumor growth, being highly expressed in gastric cancer cells, where it is activated by abnormally high Ca2+ concentrations.11) This leads to activation of AKT and β-catenin, thereby promoting cancer cell growth.12) The TRPV family also functions in a variety of immune cells.13,14) In macrophages, the TRPV1 channel regulates the expression of genes involved in the inflammatory response.13) In T cells, inhibition of the TRPV1 and TRPV4 channels suppresses T cell activation and induces release of cytokines such as IFNγ, IL-2, and TNF.14) However, little is known about TRP channels in MDSCs. In this study, we focused on the TRPV4 channel and examined its influence on the differentiation of BMCs to MDSCs, and on the function of MDSCs.
Recombinant mouse GM-CSF was purchased from Biolegend (San Diego, CA, USA). Recombinant mouse IL-6, FITC-conjugated anti-CD11b mAb and PE-conjugated anti-Gr-1 mAb were purchased from Tonbo Bioscience (San Diego, CA, USA). 2’,7’-Dichlorodihydrofluorescein diacetate (H2DCF-DA) was purchased from Sigma-Aldrich (St Louis, MO, USA), and DAF-FM DA was purchased from Goryo Chemical, Inc. (Hokkaido, Japan). GSK1016790A (a TRPV4 channel agonist) and RN-1734 (a selective TRPV4 channel antagonist) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Rabbit anti-STAT3 (Tyr705) (D3A7) mAb, rabbit anti-phospho-STAT3 (D3Z2G) mAb and other secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
AnimalsMale C57BL/6 mice were purchased from Sankyo Labo Service (Tokyo, Japan) and used at 6-8 weeks of age. They were housed in plastic cages with paper chip bedding and bred in rooms kept at 23 ± 2°C with a relative humidity of 55 ± 10% on a 12 h light-dark cycle. They had free access to tap water and normal diet, CE-2 (CLEA Co). The mice were treated and handled according to the Tokyo University of Science’s institutional ethical guidelines for animal experiments and with the approval of Tokyo University of Science’s Institutional Animal Care and Use Committee (permit numbers S19005, S18007, S17007).
Generation of MDSCs from Bone Marrow CellsWe collected bone marrow cells from femur and tibia of the mice at 5-7 weeks of age. Bone marrow cells were cultured on 6-well plates at 1.5 × 106 cells/mL in RPMI1640 supplemented with 10% fetal bovine serum, penicillin (100 units/mL), streptomycin (100 µg/mL), GM-CSF (40 ng/mL) and IL-6 (40 ng/mL) for 3 d under a humidified atmosphere of 5% CO2 in air at 37°C.15) Differentiation into MDSCs were induced by treatment with IL-6 and GM-CSF.16,17) A few % of CD11b+Gr-1+ cells were present in the isolated bone marrow cells, and about 50-60% of CD11b+Gr-1+ cells were obtained after differentiation induction.
Real Time RT-PCRTotal RNA was extracted from MDSCs, and first-strand cDNA was synthesized with PrimeScript Reverse Transcriptase (Takara Bio, Shiga, Japan). The cDNA was used as a template for real-time PCR analysis: reactions were performed in a CFX Connect Real-Time System (Bio-Rad, Hercules, CA, USA). RT2-qPCR® primer assays for mouse Arg-1, iNOS and TRPV4 were purchased from Qiagen (Hilden, Germany). GAPDH mRNA was determined as an internal control. Expression of mRNA was measured as described previously.18) Each sample was assayed in a 20 µL amplification reaction mixture, containing cDNA, primers and 2 × KAPA SYBR® FAST qPCR Master Mix (Kapa Biosystems, Wilmington, MA, USA). The amplification program included 40 cycles of two steps (95°C and 60°C, respectively). Fluorescent products were detected at the last step of each cycle. The obtained values were within the linear range of the standard curve and were normalized to GAPDH mRNA expression.
Flow CytometryCells were washed with RPMI 1640-based buffer19) and stained for 30 min at 4°C with FITC-conjugated anti-CD11b mAb or PE-conjugated anti-Ly-6G (Gr-1) mAb. The cells were washed twice with RPMI buffer, and assessed by FACScalibur flow cytometer (BD Biosciences, Franklin, New Jersey, USA). Data analysis was done with Flow Jo software (Tree Star).
ImmunoblottingCells (3.0 × 106 cells/mL) were lysed, and the lysate was mixed with 2 × Laemmli sample buffer (Bio-Rad Laboratories) and 10 mM dithiothreitol, and incubated at 95°C for 10 min. Aliquots were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and bands were transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was incubated at 4°C overnight in TBST (0.1% Tween-20, 10 mM Tris-HCl, 0.1 M NaCl) containing 1% BSA, then further incubated overnight at 4°C with phospho-STAT3 (1:2000) or STAT3 (1:1000) (Cell Signaling Technology, Beverly, MA, United States). Membranes were washed with TBST for 30 min, incubated with goat horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:20,000) for 1.5 h at room temperature, and washed again with TBST for 30 min. Specific proteins were visualized by using ImmunoStar®LD (FUJIFILM Wako Pure Chemical Corporation). Bands were analyzed with Image Studio 4.0 for C-DiGit Scanner (LI-COR).
StatisticsResults are expressed as mean ± SE. The statistical significance of differences between control and other groups was calculated by using Dunnett’s test or Mann-Whitney rank sum test. Calculations (Dunnett’s test) were done with the Instat version 3.0 statistical software package (GraphPad Software, San Diego CA). The criterion of significance was set at P < 0.05.
We first investigated the expression of the TRPV4 channel in BMCs and MDSCs using real-time RT-PCR. TRPV4 channel mRNA was expressed in both BMCs and MDSCs (Fig. 1). Although TRPV4 channel expression has been reported to be independent of the differentiation of bone marrow-derived dendritic cells (BMDCs),20) we found that the TRPV4 channel was expressed at a higher level in BMCs than in MDSCs.
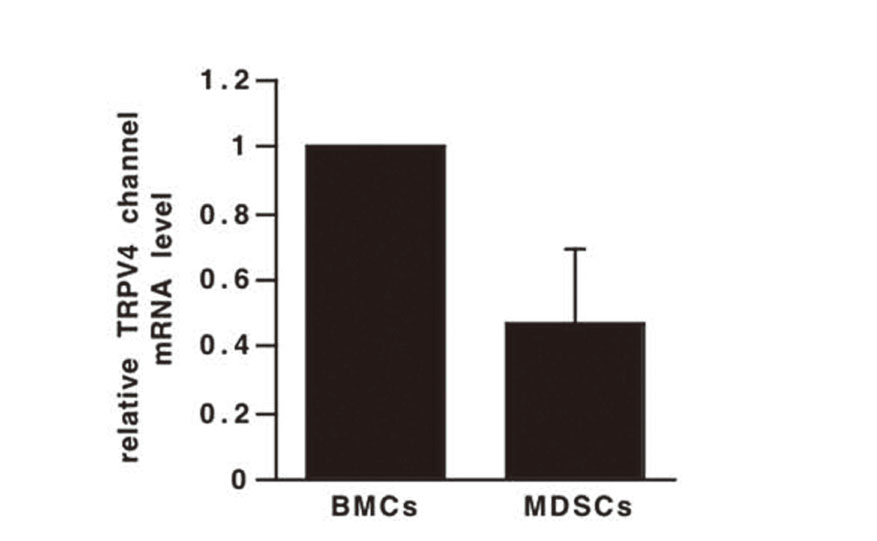
TRPV4 Channel Expression in BMCs and MDSCs
MDSCs were generated from mouse BMCs and incubated for 3 d. Expression of TRPV4 mRNA in BMCs and MDSCs was quantified using real-time RT-PCR. Each value represents the mean ± S.E. (n=3).
Next, we investigated the effects of an agonist and an inhibitor of TRPV4 channel on the differentiation of BMCs to MDSCs. MDSCs generally have been referred to as CD11b+Gr-1+ cells. Flow cytometric analysis showed that the percentage of CD11b+Gr-1+ cells was significantly increased in the presence of the TRPV4 channel antagonist RN-1734 and decreased in the presence of the TRPV4 channel agonist GSK1016790A (Fig. 2A-D, respectively). Furthermore, the inhibitory effect of GSK1016790A was attenuated by RN-1734 (Fig. 2E), suggesting that GSK1016790A-induced attenuation of differentiation to MDSCs is mediated through activation of the TRPV4 channel of BMCs. These results suggest that the TRPV4 channel regulate the differentiation of BMCs to MDSCs.
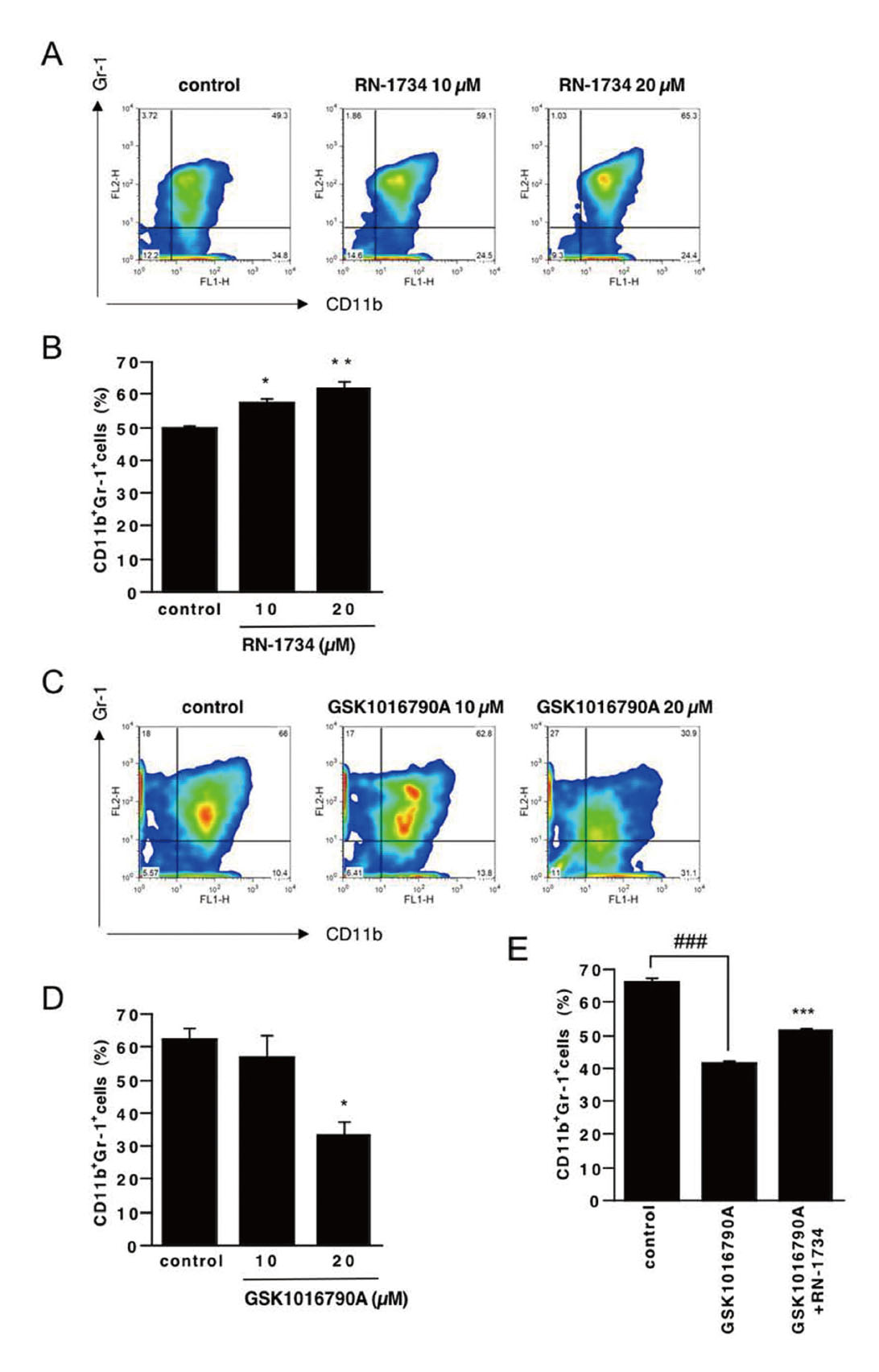
Involvement of the TRPV4 Channel in Differentiation of MDSCs
(A) MDSCs were generated from mouse BMCs in the absence (control) or presence of the TRPV4 channel antagonist RN-1734 and incubated for 3 d. A representative flow-cytometric analysis using FITC-conjugated anti-CD11b mAb and PE-conjugated anti-Gr-1 mAb is shown. (B) The percentage of CD11b+Gr-1+ cells generated in the absence or presence of RN-1734 was measured by flow cytometry. (C) MDSCs were generated from mouse BMCs in the absence (control) or presence of the TRPV4 channel agonist GSK1016790A and incubated for 3 d. A representative flow-cytometric analysis using FITC-conjugated anti-CD11b mAb and PE-conjugated anti-Gr-1 mAb is shown. (D) The percentage of CD11b+Gr-1+ cells generated in the absence or presence of GSK1016790A was measured by flow cytometry. (E) The percentage of CD11b+Gr-1+ cells generated in the presence of both 20 µM GSK1016790A and 20 µM RN-1734 was measured by flow cytometry. Each value represents the mean ± S.E. (n=3). (A-D) Significant differences between control and MDSCs treated with reagents are indicated with asterisks (* p<0.05, ** p<0.01). (E) Significant differences between GSK1016790A and GSK1016790A+RN-1734 are indicated with asterisks (*** p<0.001) and significant differences between control and MDSCs are indicated with asterisks (### p<0.001).
Two important factors that contribute to the suppressive activity of MDSCs are Arg-1 and iNOS; they promote L-arginine depletion and suppress T cell proliferation and function.21,22) Therefore, we investigated whether the TRPV4 channel agonist GSK1016790A affects the expression of Arg-1 and iNOS in MDSCs using real-time RT-PCR. Expression of Arg-1 and iNOS was decreased by GSK1016790A as compared with the control (Fig. 3). These results suggest that the TRPV4 channel agonist attenuated the immunosuppressive ability of MDSCs.
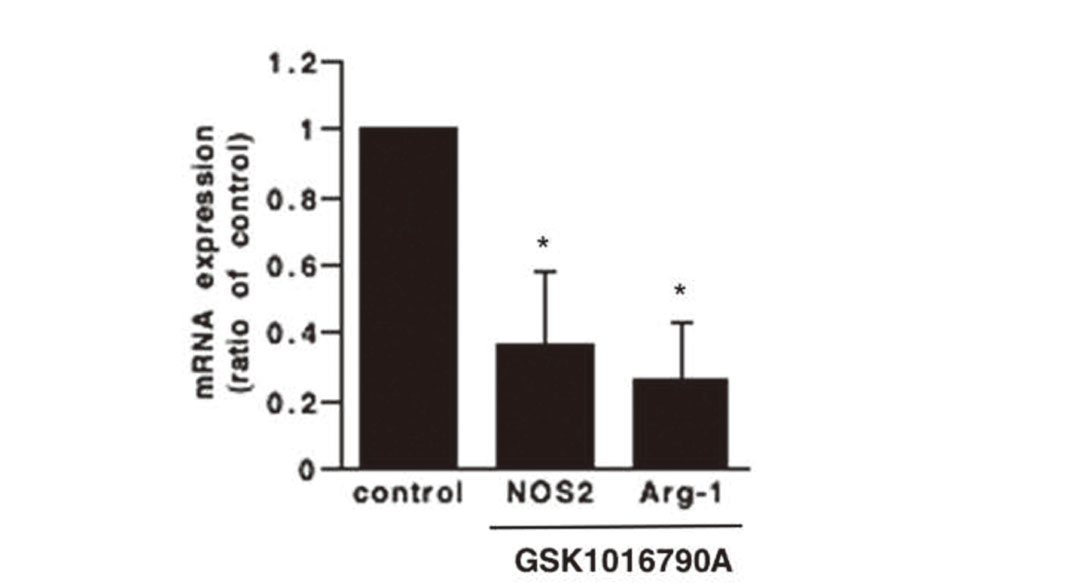
Effects of the TRPV4 Channel Agonist GSK1016790A on the Expression of Arg-1 and iNOS in MDSCs
Quantitative PCR analysis of NO synthase 2 (NOS2) and arginase 1 (Arg-1) mRNA expression in MDSCs. MDSCs were generated from mouse BMCs in the absence (control) or presence of 20 µM GSK1016790A and incubated for 3 d. The control group was used as reference and assigned to 1. Each value represents the means ± S.E. (n=3). Significant differences between control and MDSCs treated with GSK1016790A are indicated with asterisks (* p<0.05).
The main immunosuppressive pathways of MDSCs are production of ROS and NO, which suppress T cell proliferation.2) Therefore, we investigated whether TRPV4 channel affects ROS and NO production by MDSCs. Flow cytometric analysis showed that treatment with GSK1016790A increased ROS production (Fig. 4A and B), as well as NO production (Fig. 4C and D). These results are inconsistent because the number of MDSCs was reduced by GSK1016790A in Fig. 2. MDSCs are immature and heterogeneous bone marrow cell populations, and some have been reported to differentiate into mature macrophages and dendritic cells in the presence of appropriate cytokines.23–25) We hypothesized that activation of TRPV4 channel on BMCs promoted differentiation into other cells, which produce ROS and NO. We measured the population of F4/80+ cells in all cells treated with GSK1016790A employing anti-F4/80 antibody as a macrophage marker. Flow cytometric analysis showed that the percentage of F4/80+ cells was increased in the presence of GSK1016790A compared to the control (Fig. 5). It is reported that the phenotype of M-MDSCs in mice is Ly-6G- and F4/80med/high.26) Though it is unknown whether the F4/80+ cells shown in Fig. 3 are Ly-6G- cells, if they are Ly-6G-F4/80+ cells, they may be M-MDSCs. However, since the expression of iNOS and Arg-1 decreased in Fig. 3, we assume that F4/80+ cells would be macrophage-like cells rather than M-MDSCs.

Effects of the TRPV4 Channel Agonist GSK1016790A on the Production of NO and ROS in MDSCs
MDSCs were generated from mouse BMCs in the absence (control) or presence of GSK1016790A and incubated for 3 d. (A, C) A representative flow-cytometric analysis of the production of NO and ROS in MDSCs in the absence or presence of GSK1016790A is shown. The blue line represents the control, the green line represents MDSCs treated with DMSO, and the red line represents MDSCs treated with GSK1016790A. (B, D) Quantification of mean fluorescence intensity due to NO and ROS in MDSCs generated in the absence or presence of GSK1016790A. Each value represents the mean ± S.E. (n=3). Significant differences between DMSO and MDSCs treated with GSK1016790A are indicated with asterisks (* p<0.05, *** p<0.001).
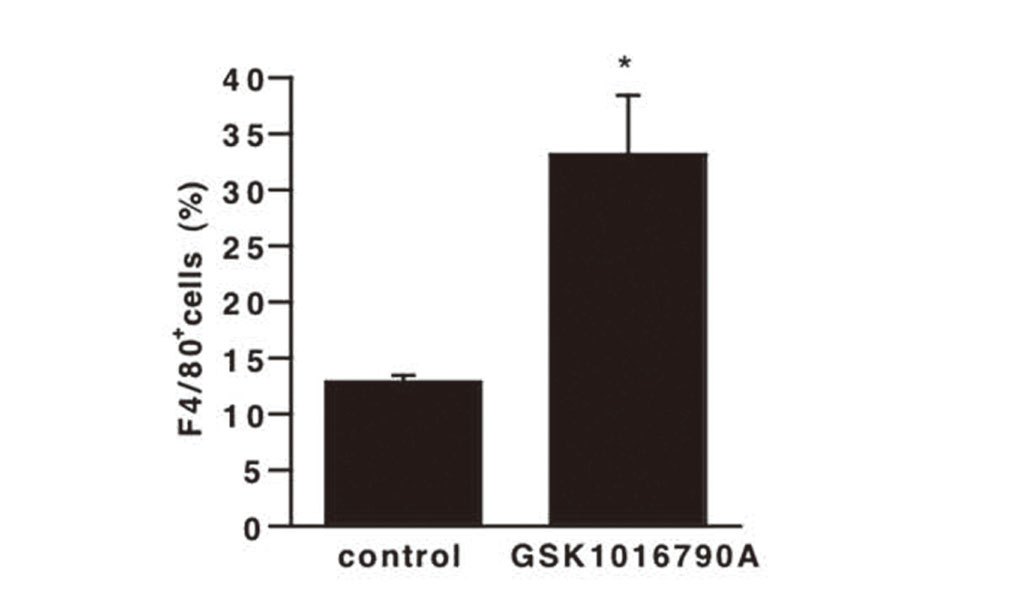
Effects of the TRPV4 channel agonist GSK1016790A on differentiation of BMCs to macrophages
The percentage of F4/80+cells generated in the presence of 20 µM GSK1016790A was measured by flow cytometry. Each value represents the mean ± S.E. (n=3). Significant differences between the control and MDSCs treated with GSK1016790A are indicated with asterisks (* p<0.05).
The transcription factor STAT3 is involved in cell survival, proliferation, differentiation, and apoptosis,27) and may be crucial for MDSC differentiation, survival, and function.28–30) STAT3 inhibition suppresses MDSC differentiation and production of Arg-131) and ROS,32) and promotes antitumor immunity.33) Therefore, we compared STAT3 activation in BMCs extracted from bone marrow and in differentiated MDSCs by means of immunoblotting. STAT3 phosphorylation was observed in MDSCs (Fig. 6), and the STAT3 phosphorylation was decreased by GSK1016790A (Fig. 6). These results suggest that STAT3 exists downstream of the TRPV4 channel and regulates the activity of MDSCs. IL-6-dependent JAK-STAT signaling pathway activation is regulated by members of the suppressor of cytokine signaling (SOCS) protein family,34) and STAT3 phosphorylation is inhibited by SOCS1/SOCS3 upregulation.35,36) Furthermore, SOCS3 deletion in bone marrow cells results in higher levels of MDSCs in prostate tumors.37) In addition, SOCS3 is upregulated by TRPV4 channel activation.38) Therefore, TRPV4 channel activation by GSK1016790A might activate SOCS3 and suppress STAT3 phosphorylation, resulting in the suppression of BMCs differentiation to MDSCs, as well as inhibition of MDSCs function.
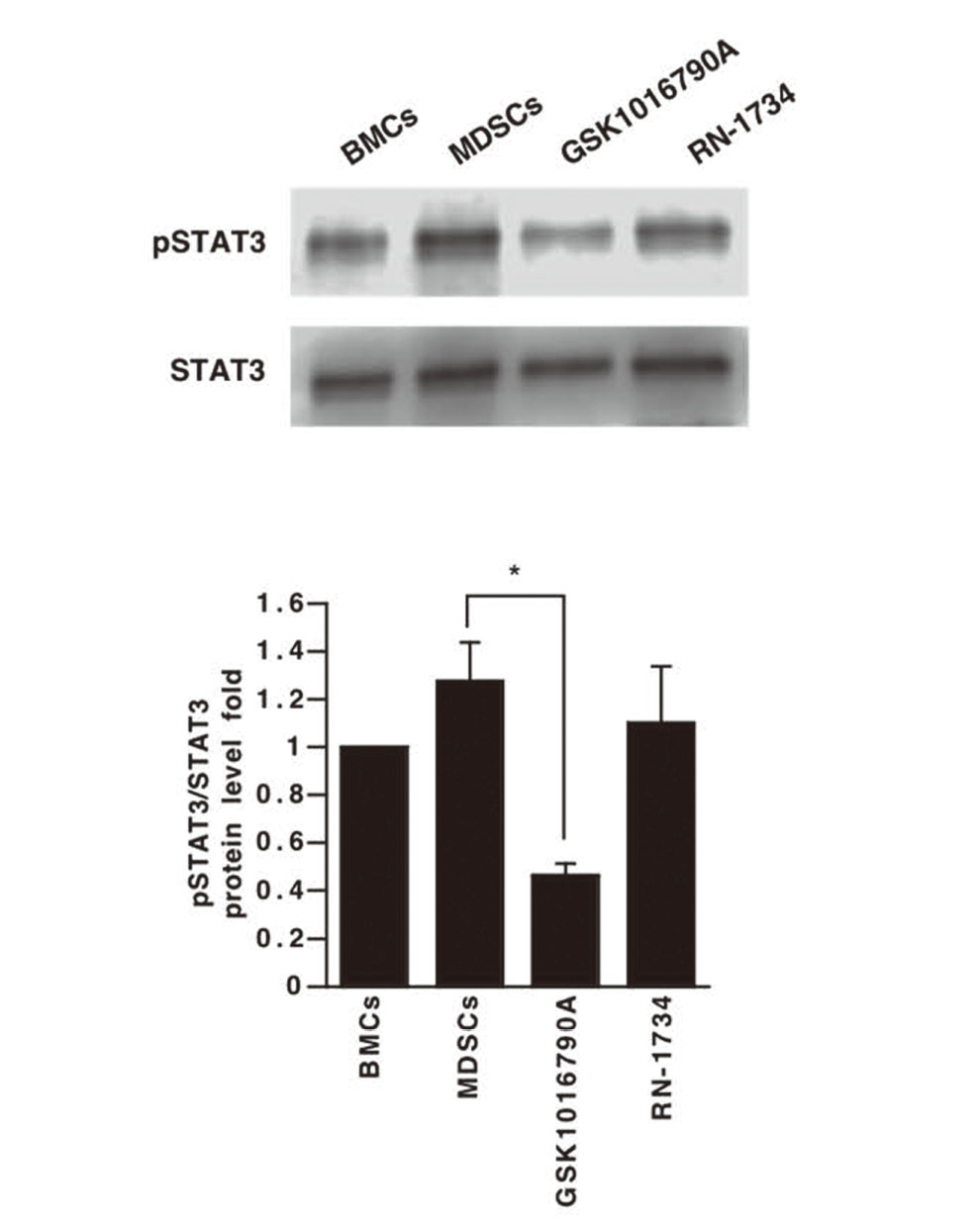
Effect of the TRPV4 Channel Inhibitor RN-1734 and the Agonist GSK1016790A on STAT3 Phosphorylation
MDSCs were generated from mouse BMCs in the absence or presence of 20 µM GSK1016790A or 20 µM RN-1734 and incubated for 3 d. Phosphorylation of STAT3 was analyzed by immunoblotting. The density of target bands was normalized to that of the corresponding total protein (STAT3). Typical data of 3 independent experiments are shown on upper side. Each value represents the mean ± S.E. (n=3). Significant differences between MDSCs and MDSCs treated with reagents are indicated with asterisks (* p<0.05).
In conclusion, our findings indicate that the TRPV4 channel agonist GSK1016790A suppresses not only the differentiation of BMCs to MDSCs, but also the immunosuppressive ability of MDSCs through attenuation of STAT3 activation. TRPV4 channels in MDSCs could be a promising target for cancer immunotherapy.
Conflict of interestThe authors declare no conflict of interest.