2021 Volume 4 Issue 1 Pages 27-35
2021 Volume 4 Issue 1 Pages 27-35
Enterocytes in neonatal rodent endocytose milk materials as macromolecule and digest them into small nutrient molecules. Bulk endocytic membrane flow sustaining nutrient uptake inevitably brings the plasma membrane into large lysosomes, as known as supranuclear vesicles or apical vacuoles which exhibit a unique morphology in infant enterocytes. Endocytic delivery to the large vacuoles required the function of Rab7 GTPase. The Rab7-deficient neonatal enterocytes became filled with abnormal gigantic vacuoles as they migrated from the intervillus pocket to the distal region of the villi and they became defective in taking up macromolecules. Infant animals lacking Rab7 in enterocytes exhibited growth retardation. These results showed that the Rab7-dependent endocytic pathways play an important role in nutrient absorption during pre-weaning growth.
The endocytic pathway constitutes a major route for delivering cell surface and extracellular macromolecules to the cell interior. Initially, a portion of the plasma membrane is pinched off to form the endocytic vesicle. The endocytic vesicles are then converted into early and late endosomes and they are finally delivered to lysosomes. This highly dynamic process is sustained by an elaborate machinery that includes Rab and Arf small GTPases; the coat proteins, tethering molecules; and SNARE proteins that guarantee the specificity of vesicle delivery, thus maintaining organelle identity and cellular integrity.
Rab7 is a small GTPase that belongs to the Rab family and controls membrane dynamics at the late stages of the endocytic pathway.1) Rab7 function is essential for establishing the subcellular architecture of endocytic compartments in peri-gastrulation embryos.2,3) The visceral endoderm (VE) of mouse gastrulae is an epithelium surrounding the embryo proper. It provides a physical barrier for the embryo proper and simultaneously participates in the regulation of multiple morphogenic signals and nutrient transfer between the maternal circulation and the fetus. The VE develops large apical vacuoles serving the degrative function. Rab7 is required for the assembly of the large apical vacuoles and its absence causes accumulation of numerous small vesicles, which morphologically resembled endosomes.2) Rab7-deficient embryos were defective in endocytic clearance of Wnt antagonist Dkk1 by the VE cells and thereby, failed to establish correct spatial patterning of Wnt activity.3)
The intestinal polarized epithelial cells, enterocytes, share functional and morphological similarities with the VE cells. The enterocytes are functioning in digestion and absorption of nutrients, and also acting as a barrier to microorganism, toxins and antigens. The infant intestine is functionally and structurally different from those in adults. Neonates do not digest proteins in stomach, rather, milk proteins are delivered to the intestine undigested and infant enterocytes take up these nutrients as macromolecules by endocytosis via multiligand receptor Cubilin/Megalin/Amnionless complex.4,5) Therefore, active endocytosis may be a prerequisite for the growth of suckling animals.
This study addresses physiological significance of the highly active endocytosis in enterocytes of suckling mice. Endocytic delivery in the neonatal intestine required the function of Rab7. Mutant suckling animals with the intestine-specific deletion of Rab7 exhibited growth retardation (failure to thrive) during pre-weaning development.
All animal procedures were approved by the Ethics Committees of DWC and were performed in accordance with the institutional and national guidelines. C57Bl/6 and ICR mice were purchased from SLC Japan (Shizuoka, Japan). The animals were supplied with food and water ad libitum.
GenotypingMouse genotype was determined by PCR amplification, as described previously.2) After morphological observation, the intestine samples were incubated in 40-80 µL of QuickExtract solution (Epicentre, Madison, WI, USA) at 55˚C for 90 min and then heat inactivated at 95˚C for 15 min. Two microliters of intestine lysate were used for each PCR, in a 50 µL volume. The PCR products were analyzed by agarose gel electrophoresis. The primer pairs used are specified in Table S1.
AntibodiesRabbit or chick anti-SNX1, Rab7 and anti-Syntaxin7 antibodies were prepared as described previously.6-8) The rat anti-Lamp2 antibody was obtained from the Developmental Study Hybridoma Bank. FITC-, Cy3-, Cy5-, and horseradish peroxidase-conjugated donkey antibodies were obtained from Jackson ImmunoResearch (West Grove, PA, USA).
ImmunoblottingP2 intestinal segments were cut open longitudinally using eye surgical scissors to expose the luminal side and intestinal villi were separated from muscle layers using a 300 µm nylon mesh. The villi were then collected and lysed in a lysis buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid, 1% sodium dodecyl sulfate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche, Basel, Switzerland). Cell debris was removed by centrifugation and supernatants were processed for immunoblotting, as previously described.8)
Tissue HistologyPups were euthanized by decapitation and intestines were dissected and fixed overnight with 4% paraformaldehyde (PFA) in Dulbecco’s phosphate-buffered saline (PBS). Intestinal tissues were collected, dehydrated, and embedded in Technovit 7100, as described previously.8) Sections were prepared with a microtome at a thickness of 3~4 µm, and were stained with hematoxylin and eosin, as previously described.8) Electron microscopy was carried out by Tokai Electron Microscopy, Inc. Samples were fixed with 2% PFA and 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4˚C overnight, washed and post-fixed in 2% osmium tetroxide for 2 h and then embedded in resin. The sections were prepared at a thickness of 70 nm and stained with uranyl acetate and lead stain solution (Sigma-Aldrich, St Louis, MO, USA). The stained sections were observed under a JEM-1400 Plus electron microscope (JEOL, Tokyo, Japan) and images were captured with a CCD camera (VELETA; Olympus, Tokyo, Japan).
Immunofluorescent Labeling of the IntestineImmunofluorescent staining was performed as previously described.2) For immunohistochemistry, intestines were fixed overnight with 4% PFA at 4˚C, then tissues were incubated with primary and secondary antibodies in a blocking solution containing 0.5% Triton X100, 0.5% TSA blocking reagent (PerkinElmer) and 1% normal donkey serum in PBS. The labelled tissues were mounted with VECTASHIED containing DAPI (Vector Laboratories) and visualized under a confocal laser scanning microscope (Zeiss LSM800, Oberkochen, Germany).
Intestine Culture and Endocytic LabelingIntestines were dissected out and placed in Dulbecco’s modified Eagle medium (DMEM) containing 584 mg/L L-glutamine and 110 mg/L sodium pyruvate. The intestinal tracts were cut into 2~3 mm lengths in DMEM. The intestinal segments were cut open longitudinally using eye surgical scissors to expose the luminal side. The intestinal tissues were then cultured in a 1:1 mixture of DMEM and rat serum under 5% CO2 and 95% air at 37˚C for 30 min. The intestinal tissues were incubated with 2 mg/mL FITC-dextran (70,000 MW, lysine-fixable, Invitrogen) or tetramethylrhodamine-dextran (70000 MW, lysine-fixable, Invitrogen) in culture medium for the indicated times and then washed with DMEM. After the final labeling and chase, intestinal tissues were transferred to 4% PFA/PBS, fixed overnight at 4˚C, and then processed for observation under a laser-scanning confocal microscope (Zeiss LSM800).
Body WeightThe body weight of male and female mice was monitored from birth and at the indicated times. E19.5 pups were removed from mothers and their body weight was measured. Their sex was determined by PCR using a primer set Zfy-Fw2 and Zfy-Rv2 (Table S2).
Statistical AnalysisStudent’s unpaired t-test was used to analyze the differences between two groups. Values were regarded as significant at P < 0.05. Data are expressed as means ± SEM.
The expression of Rab7 in the neonatal (postnatal day 2, P2) intestine were examined using whole-mount immunofluorescence microscopy. Strong Rab7 signals were observed in the apical region of intestinal epithelial cells (enterocytes) in the intestines of wild-type mice (Fig. 1a, arrows), whereas Rab7 was seen at markedly lower levels in the lamina propria beneath the epithelium (Fig. 1a and b). The accumulation of large quantities of the endosomal small GTPase, Rab7, was consistent with the vigorous endocytosis in enterocytes.

Intestinal Epithelial Cell-Specific Knockout of Rab7
Whole-mount immunofluorescent staining of P2 intestines of Rab7fl/fl mice (a and b). Rab7 (red) signals were observed in the apical region of enterocytes (arrows) and nuclei stained with DAPI (blue) are also shown. The Rab7 signals in Rab7fl/fl VilCreTg/+ intestinal enterocytes are below detectable levels (c and d). Western blotting analysis of Rab7 and β-actin using the mucosa lysates prepared from P2 Rab7fl/fl (n = 2, lane 1 to 2) and Rab7fl/fl VilCreTg/+ mice (n = 3, lane 3 to 5) (e). The relative amounts of Rab7/β-actin determined from the immunoblots shown in e were measured and shown in f. X-gal staining of E19.5 intestinal tissue of Rab7fl/fl Gt(ROSA)26Sortm1Sho (g) and Rab7fl/fl VilCreTg/+ Gt(ROSA)26Sortm1Sho mice (h). Bar indicates 20 µm in panels a to d, and 500 µm in g and h. The error bars denote the SEM, and p value is shown in the graph.
Given the preferential expression of Rab7 in enterocytes, I examined its role in the intestinal epithelium. Because systemic deletion of Rab7 function leads to developmental arrest at early embryogenesis,2) enterocyte-specific Rab7-deficient mice were generated. In the VilCre mouse line, Cre recombinase is expressed specifically in the intestinal epithelium from 12.5 d post coitum (dpc).9) The VilCre transgene was introduced into Rab7fl/fl mice (Fig. S1).2) This Cre-mediated recombination between the two lox segments deletes the exons 2 (containing the ATG start codon) and 3, thereby generating a null allele. Rab7fl/fl VilCreTg/+ mice were born and survived into maturity at the expected Mendelian ratios (Rab7fl/fl: Rab7fl/fl VilCreTg/+ = 247 (52.5%): 223 (47.5%) and were fertile. In Rab7fl/fl VilCreTg/+ mice, Rab7 was below detectable levels in enterocytes (Fig. 1c and d). Immunoblotting analysis confirmed the decrease of Rab7 protein in mutant P2 intestinal mucosa (Fig. 1e and f). Rab7fl/fl VilCreTg/+ mice with the transgenic reporter line, Gt(ROSA)26Sortm1Sho, which expresses a β-galactosidase-neomycin phosphotransferase fusion gene (βgeo), after Cre-mediated excision of the loxP-flanked DNA sequence,10) expressed LacZ in almost all enterocytes by X-gal staining at E19.5 in mice carrying VilCre transgene. By contrast, LacZ-positive enterocytes were not observed in mice without the VilCre transgene (Fig. 1g and h). The Rab7 protein levels at E19.5 were also verified using immunoblotting (Fig. S2). These results indicated that the Cre-mediated deletion efficiently abolished the expression of Rab7 in the intestinal epithelium.
Vacuolation of Neonatal Enterocytes in Mice Lacking Rab7There were no obvious differences in the macroscopic appearance of the intestines dissected from Rab7fl/fl VilCreTg/+ (termed cKO) and control mice (Rab7fl/fl). In hematoxylin and eosin (H&E) stained Technovit sections of neonates (P2), the subcellular structures appear distinctive (Fig. 2). The enterocytes of control neonatal intestines contained large lysosomes, as known as supranuclear vesicles or apical vacuoles11-13) filled with eosinophilic contents (Fig. 2a-c). By contrast, the apical compartments in cKO mutant enterocytes exhibited extensive vacuolation (Fig. 2d-f). The swollen apical compartments varied in size and accumulated fewer membrane-bound vesicles inside. These abnormal gigantic vacuoles filled most of the apical cytoplasmic space of Rab7-deficient enterocytes (Fig. 2f).

Pathological Vacuolation of Neonatal Enterocytes in Mice Lacking Rab7
Hematoxylin and eosin (H&E) staining of Technovit-embedded sections of intestines. Magnified images of boxed regions are shown. H&E staining of the ileum of control mice (a to c) and cKO mice (d to f) at postnatal day 2 (P2). Arrowheads indicate the large apical vacuole in the control enterocytes and arrows indicate the abnormal vacuolation observed in Rab7-deficient enterocytes. Bars, 50 µm.
The subcellular structures of enterocytes were examined by transmission electron microscopy. Large membrane-limited organelles with high electron density were prominent at the apical side of the nuclei in control enterocytes (Fig. 3a and e). A tubulo-vesicular network, presumably a complex of endocytic compartments, was present in the apical cytoplasm just below the microvilli (Fig. 3c, yellow arrows).14) Knockout of Rab7 resulted in a decrease in the number of electron-dense apical vacuoles and instead, the cKO enterocytes were replaced by swollen vacuolar structures with lower electron density (Fig. 3b, f, and g). Morphometric analyses on the electron micrograph confirmed that the swollen vacuoles were larger, but appeared less dense contents than the large apical vacuoles in control enterocytes (Fig. 3h to k). Hereafter, these vacuolated structures are referred as abnormal gigantic vacuoles. The network of endosomes beneath the apical brush border were also swollen (Fig. 3d, red arrows). No significant difference in microvilli structure between control and cKO neonates was observed (Fig. 3c and d).

Ultrastructural Examination of Rab7-Deficient Enterocytes
Electron microscopic images of the ileum from neonatal control (a, c and e) and cKO pups (b, d, f and g) at P2 are shown. The large apical vacuoles (AV) with dense electron density in control enterocytes are indicated. The asterisks (*) indicate the abnormal gigantic vacuoles observed in Rab7-deficient enterocytes. The tubulo-vesicular network in control enterocytes is shown by yellow arrows (c), and this early endosome network was swollen in cKO cells (d, red arrows) .The area and electron density distributions of the intracellular vesicles in control enterocytes (n = 23, selected as shown in h) and that of gigantic vacuoles in cKO cells (n = 18, selected as shown in i) were measured on electron microscopic images and shown in Scatterplot (j). The distribution of vesicles (> 1 µm2) are shown in k (statistical significance was evaluated by two-tailed Student’s t-test). The cKO enterocytes accumulated gigantic swollen vacuolar structures with lower electron density (as shown by asterisks). AV, large apical vacuoles; N, nuclei of enterocytes; Mv, microvilli. Bar indicates 20 μm in panels a and b, 1 μm in c and d, 10 μm in e to g, and 5 μm in h and i. Horizontal bars represent the mean ± SEM and p value is shown in the graph.
The expression patterns of various endosomal and lysosomal marker molecules in enterocytes were examined (Fig. 4). The apical vacuoles in control enterocytes were positive for Lamp2, a lysosomal marker protein, confirming that large apical vacuoles were formed in enterocytes at P2 and shared a common feature with lysosomes, as previously reported15-18) (Fig. 4a and b, e and f). Rab7 signals partially overlapped with signals for the early endosomal marker, sorting nexin1 (SNX1), which was localized at the apical region (Fig. 4g and h, arrowheads). The majority of Rab7 was localized to the cytoplasm, between the SNX1-positive region and the apical vacuoles positive for Lamp2 (Fig. 4e-h). Rab7 was also present in the Lamp2-positive apical vacuoles, but unlike Lamp2, Rab7 was distributed in a dot-like pattern in the limiting membrane of the apical vacuoles (Fig. 4f, g, n, and o, arrows). Syntaxin 7 (Stx7), which is involved in endocytic trafficking from early endosomes to late endosomes and lysosomes, mainly localized at the apical region, and partially overlapped with signals of Rab7 or Lamp2 (Fig. 4i and j, and m-p, arrows). In cKO enterocytes, the apical fluorescence signals of SNX1, were significantly lower than those observed in control enterocytes (Fig. 4c and d). The signals of Stx7 were detected at the apical region, but not observed on the gigantic vacuoles (Fig. 4k and l). The abnormal gigantic vacuoles in Rab7-deficient enterocytes were negative for early endosomal and lysosomal markers.

Rab7 was Required for the Organization of Late Endocytic Compartments in Enterocytes
Localization of endocytic markers, SNX1 (red), Lamp2 (green), and Rab7 (blue) in control (a and b) and cKO enterocytes (c and d). The higher magnification images of the boxed area of control enterocytes of b are shown in e to h. Lamp2 and SNX1 signals were below detectable levels in cKO enterocytes. Localization of Stx7 (red), together with Lamp2 (green) and Rab7 (blue) are shown in i to p. The higher magnification images of the boxed area of control enterocytes of j are shown in m to p. Arrows and arrowheads indicate the overlapping of organelle markers. Bar indicates 20 µm in panels a to d and i to l, and 5 µm in panels e to h, and m to p.
The intestinal enterocytes of newborn animals take up nutrients from the intestinal lumen via endocytosis. This uptake can also be observed in vitro, under appropriate culture conditions. P2 mouse intestines were cultured in the presence of rhodamine-dextran (RD), a fluorescent tracer of endocytosis, for 15 min, and then incubated for 15 min in the absence of the tracer (Fig. 5). During this chase incubation, most internalized RD was transported to the apical vacuoles (Fig. 5a). The intestines were then further labelled with fluorescein-dextran (FD) for 5 min and chased again in the absence of the fluorescent dye. Immediately after the second labelling (Fig. 5a and d), FD signals were observed in the cytoplasm close to the apical cell surface (Fig. 5a, 0 min), whereas RD reached the apical vacuoles and thus, the green and red signals were well separated. Within the next 15 min, the apical FD signals became spherical (Fig. 5b and e). Further mixing of the red (RD) and green (FD) signals occurred 15 to 30 min after the second labelling, resulting in the apical vacuoles becoming yellow (Fig. 5b and e, c and f). These observations suggested that the endocytosed materials accumulated in the tubulo-vesicular network first, in spherical compartments within ~15 min, and were then delivered to the apical vacuoles after ~30 min. Compared to its uptake in control neonatal intestines, only several faint fluorescent signals were observed between the apical plasma membrane and the abnormal gigantic vacuoles (Fig. 5g-l). As the apical vacuoles in neonatal enterocytes serves as a site for degradation of nutrients from milk, these results indicated that the endocytic uptake of nutrients in P2 Rab7-deficient intestines was impaired.

Impaired Endocytic Uptake in Rab7-Deficient Enterocytes
Endocytosis of fluorescent dextran in enterocytes. P2 intestines were cultured and labeled with rhodamine dextran (RD, red) for 15 min, chased for 15 min, pulse-labeled with fluorescein dextran (FD, green) for 5 min, and then chased for the indicated durations. The stained intestines were fixed, stained with DAPI (blue). Immediately after the second labeling, FD appeared close to the apical surface of control enterocytes (a), the FD signals became spherical within 15 min (b), and then mixing of vesicles with the large apical vacuole occurred and most large apical vacuoles became yellow (c). The merged images with differential interference contrast (DIC) are shown in d to f. However, endocytic uptake in P2 cKO enterocytes was significantly reduced (g to l). The merged images with DIC are shown in j to l. The schematic image of the uptake experiment is also shown (m). Bar, 20 µm.
The deletion of Rab7 caused contrasting phenotypes between the infant enterocytes and VE cells. The formation of gigantic vacuoles was observed in infant enterocytes (Fig. 1c, 2d-f), whereas the fragmentation of apical vacuoles was observed in VE cells.2) Expansion of vacuoles is rather similar to what found in VE cells defective in production of phosphatidyl inositol 3, 5-phosphate, an essential step in the early stage of endocytic pathway.19)
The abnormal gigantic vacuoles at the tip of each villus were larger than those close to the intervillus pocket in P2 cKO intestine (Fig. 2d). This observation led me to hypothesize that the ‘young’ enterocytes had differentiated from a proliferative stem cell located near the intervillus pocket, exhibiting smaller vesicular structures, instead of large apical vacuoles. I examined the morphology of intracellular vesicles of enterocytes using electron microscopy (Fig. 6). Rab7 cKO enterocytes in the vicinity of the intervillus pocket accumulated small vesicles (Fig. 6b3, b6) similarly to the control enterocytes (Fig. 6a3, a6). These vesicles showed an average size of 0.117 µm2, and 0.080 µm2 in cKO and control enterocytes, respectively (Fig 6c “Bottom”, right panel). The regions yet close to the intervillus pocket, the size of the vesicles became larger, but there was no obvious difference in cKO and control enterocytes (Fig. 6c “Near the bottom”, center panel). By contrast, the abnormal gigantic vacuoles (> 1 μm2) occupying 30.95 µm2 in average became apparent in cKO cells around the tip (Fig. 6b1 and b4), whereas the large apical vacuoles (> 1 μm2) in control enterocytes (Fig. 6a1 and a4) also acquire their sizes (7.67 µm2), but far less smaller than the abnormal gigantic vacuoles in the rab7-deficent enterocytes (Fig. 6c “Tip”, left panel, also see Fig. 3k).

Ontogeny of Vacuolation in Enterocytes of Mice Lacking Rab7
Toluidine Blue staining of intestinal villi of control mice (a) and cKO mice (b) at postnatal day 2 (P2). The electron microscopy of enterocytes near the tip of villi are shown in a1 and b1, the enterocytes near the base of villi are shown in a2 and b2, and the enterocytes at the base are shown in a3 and b3. The boxed area in a1-a3 and b1-b3 are shown in a4-a6 and b4-b6, responsively. The areas of intracellular vesicles of enterocytes were measured (Ctrl: n = 55, cKO: n = 32) and the distributions are shown in c (statistical significance was evaluated by two-tailed Student’s t-test). The vacuolated enterocytes show a gradient of severity along the villi that corresponds with their age. At the base, enterocytes from newborn mice appear normal, whereas at the tip, the oldest enterocytes appear the most vacuolated. N, nucleus; GV, abnormal gigantic vacuole. Bar indicates 50 μm in panels a and b, 10 μm in a1 to a3 and b1 to b3, and 2 µm in a4 to a6 and b4 to b6 . Horizontal bars represent the mean ± SEM and p values are shown in the graphs.
The large apical vacuole in rat enterocytes, referred to as a giant lysosome, is known to be formed after birth.20) Consistent with this, the embryonic enterocytes of E19.5 mice (a day before the delivery) lacked large apical vacuoles (Fig. 7a-c). No distinct difference was observed in the morphology of the apical compartments between the cKO embryonic (Fig. 7d-f) and the control enterocytes at the same developmental stage (Fig. 7a-c). Therefore, the expansion of apical compartments (Fig. 2d-f) occurred after birth suggesting that severe vacuolation of the abnormal gigantic vacuoles occur after the birth due to trafficking defects in endocytosis.
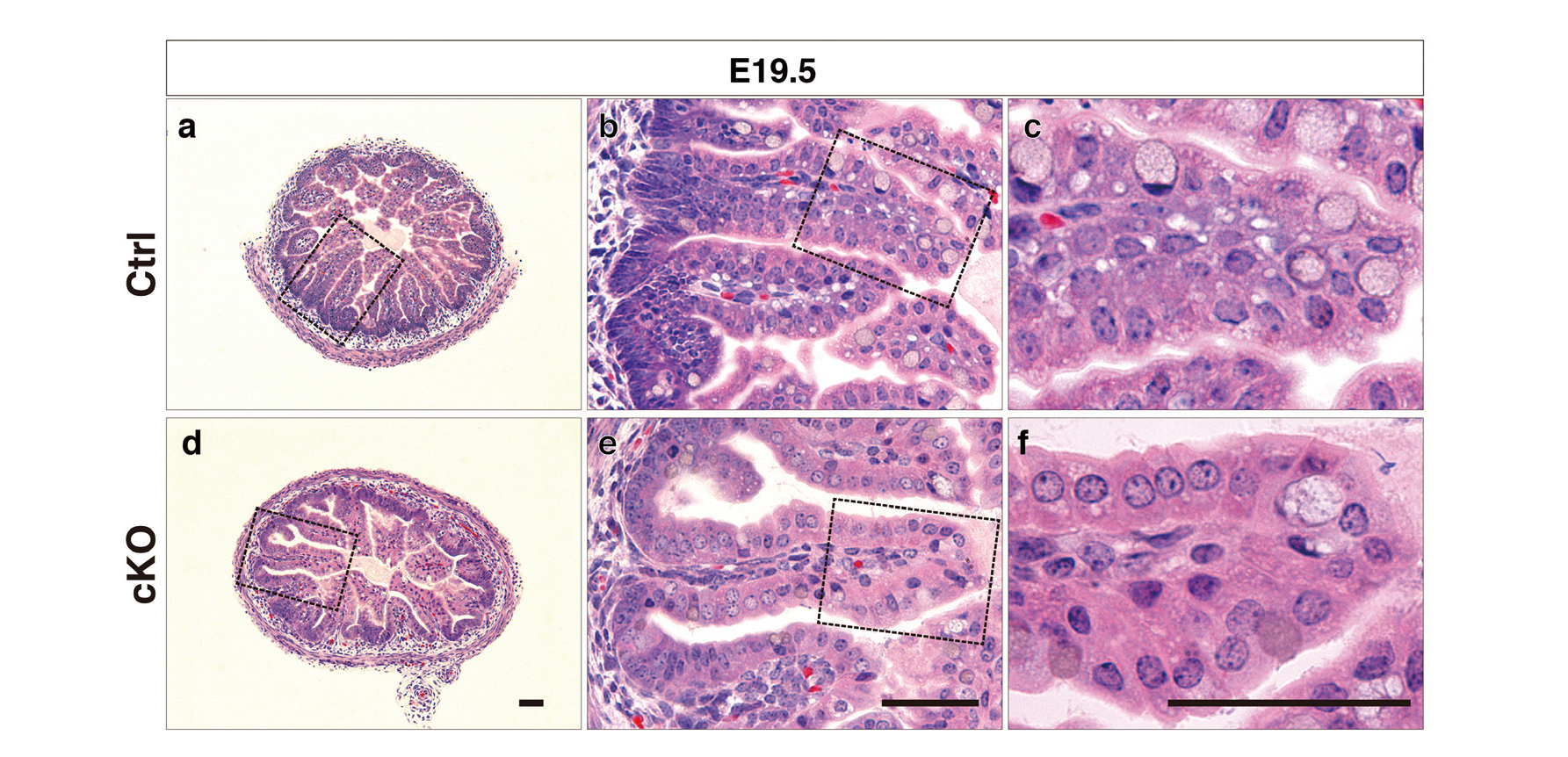
Morphology of E19.5 Embryonic Enterocytes Lacking Rab7
Hematoxylin and eosin (H&E) staining of Technovit-embedded sections of intestines and the magnified images of boxed regions are shown. H&E staining of the ileum of control embryos (a to c) and cKO embryos (d to f) at 19.5 d post coitum (E19.5) revealed no significant vacuolation. Bars, 50 µm.
Defective endocytosis in the mutant mice would cause malnourishment and thereby, affect growth. cKO mice displayed normal sucking behavior and their stomachs were filled with milk to the same extent as their littermates, indicating that their ingestion of milk was normal (Fig. 8a). Control and cKO mice displayed similar body weight at E19.5 and the day of birth. However, the weights of mutant mice became relatively smaller and this weight differential was maintained until P14 for both males and females (Fig. 8b and c). cKO mice could, despite impaired endocytic activity in enterocytes, absorb rather sufficient nutrients from maternal milk to sustain growth at a reduced rate compared with control mice.
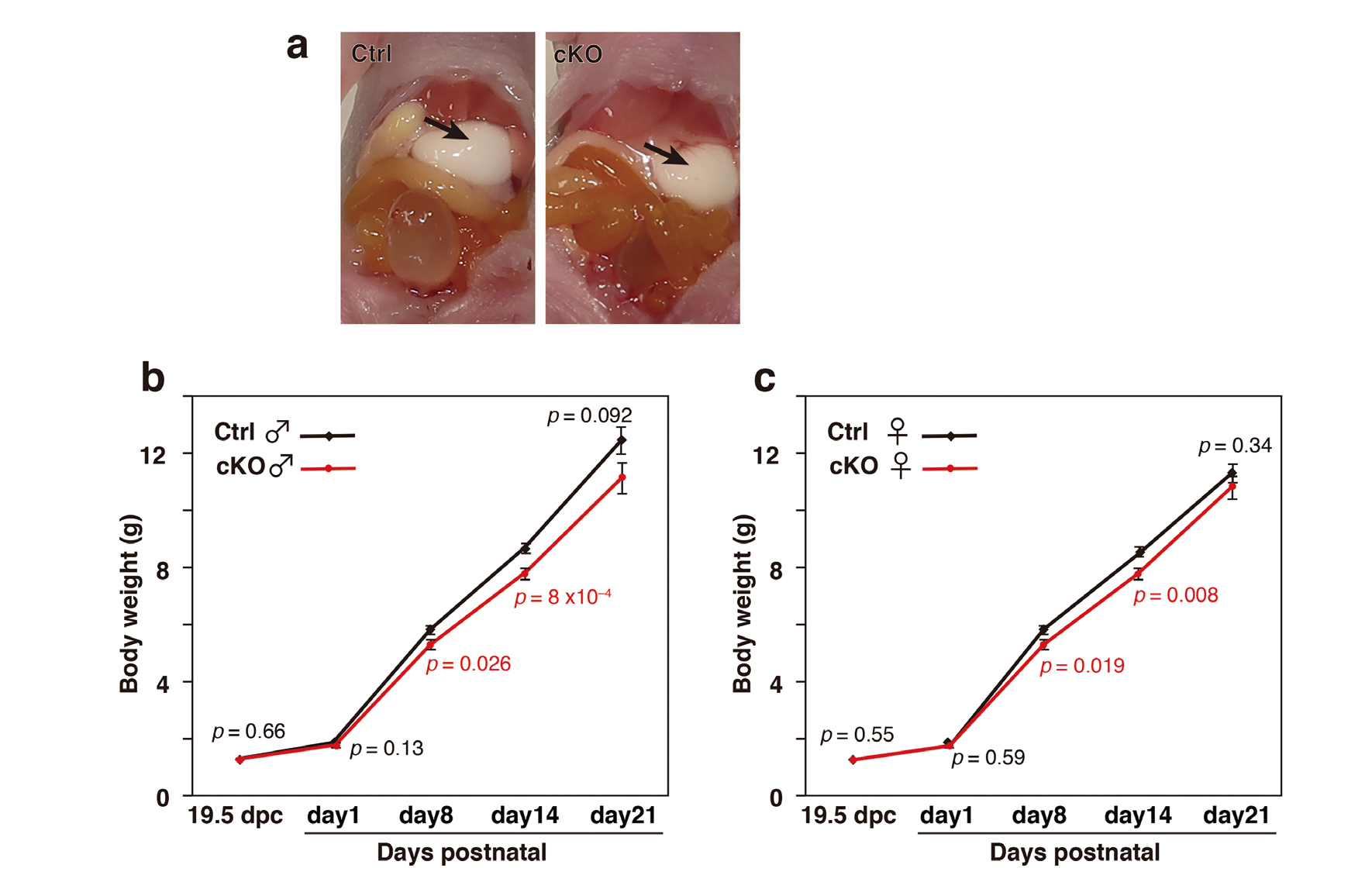
Reduced Growth in Rab7-Deficient Mice during Suckling
(a) View of a pair of litter mates at P2 shows that the stomach of the cKO pup, as well as the stomach of the normal pup, were filled with milk (arrows), indicating that suckling and ingesting were normal in cKO pups. (b) The body weight of male (for wild-type males, n = 34 [day 1], n = 17 [day 8], n = 51 [day 14], and n = 17 [day 21]; for cKO males, n = 32 [day 1], n = 11 [day 8], n = 40 [day 14], and n = 11 [day 21]) and (c) female mice (for wild-type females, n = 24 [day 1], n = 17 [day 8], n = 48 [day 14], and n = 17 [day 21]; for cKO females, n = 24 [day 1], n = 15 [day 8], n = 47 [day 14], and n = 15 [day 21]) was monitored from birth at the indicated times. E19.5 pups were removed from mothers and their body weight (wild-type males, n = 13; cKO males, n = 16; wild-type females, n = 7; and cKO females, n = 21) was measured. Sex of fetus and young pups were determined by the PCR amplification of the Zfy locus of the Y chromosome. The error bars denote the SEM and p values are shown in the graph.
It is known that intestinal digestion differs before and after weaning in mammals, including humans.21,22) In adults, extracellular hydrolases in the lumen of the digestive tract digest ingested proteins and carbohydrate polymers and then enterocytes absorb the resulting amino acids and smaller carbohydrates via specific transporters.23,24) By contrast, during suckling, milk proteins reach the intestinal lumen intact and are taken up by enterocytes via endocytosis and degraded.4) In rodents, the perinatal enterocytes generate a specialized apical endocytic complex that lasts until weaning. The apical endocytic complex consists of both tubular and vesicular endosomes adjacent to the apical plasma membrane, and large prominent vacuoles, known as supranuclear vesicles or giant vacuoles which occupy the apical cytoplasm.11-13) Morphological and histological studies have shown that these vacuoles share common features with lysosomes, including the presence of digestive enzymes, the accumulation of endocytic markers, and an acidic interior.15-18)
The visceral endoderm of embryo and infant intestine share a unique architecture of their endocytic compartments, i.e., they have unusually large lysosomal compartments. However, the impact of the loss of Rab7 appeared to be quite different. In Rab7-deficient visceral endoderm cells, the large apical vacuoles are absent, but smaller endosome-like vesicles fill the cytoplasm.2) By contrast, in the infant intestine, abnormal gigantic vacuoles develop upon the loss of Rab7. These profound differences in cellular phenotype may reflect the duration of the lack of Rab7 function. In newborn mutant pups, the enterocytes located proximal to the intervillous pockets lacked the abnormal gigantic vacuoles. These observations were consistent with the view that the initial impact of the loss of Rab7 function is the fragmentation of large apical vacuoles observed in embryo visceral endoderm cells. In the visceral endoderm, the assembly of apical vacuoles begins at E5.5~5.7, and they become apparent at E6.2~6.7, within 1 d. The half-life of enterocytes in newborn mice is estimated to be more than 5 d.25) Thus, the distally located enterocytes were Rab7-deficient for longer than the visceral endoderm, as observed in the previous study.2) Therefore, the phenotype in the enterocytes, especially at the tip of the villus, reflected the chronic effects of Rab7-deficiency that caused a general “traffic jam” at multiple stages including the initial internalization, resulting in failure of endocytic uptake and in expanding the endocytic compartments as a tertiary consequence. In contrast to the phenotype in ‘elder’ enterocytes, the embryonic enterocytes showed an immediate defect upon the loss of Rab7 function like in the visceral endoderm. Furthermore, the almost normal appearance of the ‘young’ enterocytes located close to the intervillous pockets suggested that they were still active in endocytosis, thereby sustained the nutrition of newborn animals.
Rab7 is the target of pathogens. For example, Salmonella enterica serovar Typhimurium infects enterocytes in Rab7-positive endocytic compartments and the interleukin 22-induced upregulation of Rab7 is thought to be involved in excluding the bacterium.26) Rab7 is a critical regulator of the assembly of Salmonella-containing vacuoles, where the bacterium proliferates and is thus, intimately involved in infection.27,28) Mycobacterium avium preferentially infects enterocytes. Infants are more susceptible to bacterial infection. Therefore, mutant mice with enterocyte-specific Rab7 deficiency may exhibit an increased susceptibility to microbial infections and low nutrient intake in natural circumstances.
I thank Drs Ge-Hong Sun-Wada and Yoh Wada for their instruction and discussion throughout this study.
Conflict of interestThe author declares no conflict of interest.