2021 Volume 4 Issue 5 Pages 148-154
2021 Volume 4 Issue 5 Pages 148-154
Miso is a traditional Japanese fermented food made by fermenting steamed soybean with koji (fermented cereals with Aspergillus oryzae). Many types of miso are produced in Japan, including miso with rice and/or barley depending on the region where it is produced. In this study, we used 1H NMR metabolomic analysis to compare the characteristics of the components (metabolites) of miso with different ingredients. Three types of miso were compared: soybean miso, rice miso, and barley miso. After measuring the 1H NMR of the aqueous solution of each miso, multivariate analysis of the spectral integration data was performed to compare the characteristic metabolites. Principal component analysis (PCA) showed a separation between soybean miso and rice, barley miso. Orthogonal Projection to Latent Structure Discriminant Analysis (OPLS-DA) extracted ethanol, saccharides such as glucose, and amino acids as metabolites contributing to the separation. Ethanol and glucose were higher in rice and barley miso, especially glucose in barley miso and ethanol in rice miso. Soybean miso was characterized by its high content of amino acids, including branched-chain amino acids. It was suggested that the characteristics of these ingredients were influenced not only by differences in ingredients, but also by the fermentation period and other factors. Although the number of metabolites that can be analyzed by 1H NMR metabolomic analysis is smaller than that by GC/MS or LC/MS, it does not require any pretreatment and is easy to measure, so it can be applied to the comparison of food components and quality control, as in the analysis of miso components.
Miso is a traditional Japanese fermented food. It is made from steamed soybean, salt and koji (fermented cereals or soybean by Aspergillus oryzae) and is used in various dishes as a long-term preserved food in Japan.1,2) There are some types of miso, such as soybean miso which is made by fermenting steamed soybeans with soybean koji (fermented soybean by Aspergillus oryzae) and salt, rice miso which is made by fermenting soybeans with rice koji and salt, barley miso which is made by fermenting soybeans with barley koji instead of rice koji, as well as blended miso, which is made by fermenting a mixture of rice and barley koji. Depending on the degree of fermentation, white miso, red miso, light or dark-colored miso are produced. There are also differences in taste, such as dry or sweet, due to differences in the ratio of salt and koji added. Various types of miso are produced according to usage and region, and are consumed as a basic seasoning for Japanese food.1,2) In recent years, the consumption of miso has been decreasing due to changes in dietary habits in Japan. However, the benefits of miso, such as the prevention of lifestyle-related diseases, have been reported.3,4) In addition to its nutritional value as a food, miso has been reported to have antioxidant, radical scavenging,5) anticancer6,7) and hypotensive effects8,9) in vivo, as well as effects on the intestinal microflora,10) which is currently attracting attention for its health effects.
Metabolomic analysis is a comprehensive measurement and analysis of various metabolites, amino acids, sugars, organic substances, lipids, alcohols, and other low-molecular-weight metabolites with a molecular weight of 1500 or less. It is used in various fields, such as analysis of food components, verification of the nutritional status and homeostasis of living organisms, search for biomarkers useful for diagnosis in pathological conditions, and investigation of the effects of pharmaceuticals.11–13) In metabolomics, CE/MS for ionic compounds, LC/MS for neutral compounds, and GC/MS for volatile compounds14) are often used according to the characteristics of metabolites. NMR is also used for metabolomics, because it can be easily measured without pretreatment of samples.15) The NMR metabolomic analysis has been reported to be applied in many fields, such as quality evaluation of agricultural products, drugs and foods,16–18) and prediction of changes in basal metabolism by measuring the dialysis waste fluid from dialysis patients.19–21) In this study, we compared the components of three types of miso by metabolomic analysis using 1H NMR.
Deuterium oxide (99.8%) and sodium 3-(trimethylsilyl) propionic 2,2,3,3-d4 acid (TSP) were purchased from Fujifilm Wako pure chemical Co. (Osaka, Japan). The analytical grade reagents (purity > 99%) were used.
The Miso SamplesCommercial miso products, rice miso (N = 5), soybean miso (N = 4), and barley miso (N = 3), with no food additives were analyzed in this experiment. The miso products were listed in Table 1. They were analyzed immediately after opening the package because they were affected by oxygen.
| Code | Ingredient | Name | Manufacturer | Area in Japan | |
|---|---|---|---|---|---|
| Rice miso | 1R | soybean, salt, rice-koji*1 | Genmai*2-miso | Kohzan | Hiroshima |
| 2R | Genmai-miso | Masuyamiso Co. | Hiroshima | ||
| 3R | Kome*3-miso | Hikari-miso Co. | Nagano | ||
| 4R | Kome-miso | Marukome Co. | Nagano | ||
| 5R | Kome-miso | Tsuruya Co. | Toyama | ||
| Soybean miso | 6S | soybean, salt, soybean-koji*1 | Mame-miso | Noda co. | Aichi |
| 7S | Hatcho-miso*4 | Kakukyu Co. | Aichi | ||
| 8S | Hatcho-miso | Maruya Co. | Aichi | ||
| 9S | Hatcho-miso | Noda Co. | Aichi | ||
| Barley miso | 10B | soybean, salt, barley-koji*1 | Mugi-miso-Hojun | Masuyamiso Co. | Hiroshima |
| 11B | Mugi-miso-Tokusen | Masuyamiso Co. | Hiroshima | ||
| 12B | Mugi-miso | Shinjyomiso Co. | Hiroshima |
*1 fermented serial (rice, barley or soybean) by Aspergillus oryzae. *2 Rice that has not been polished. Contains high levels of fats and minerals. *3 Polished rice. *4 A traditional miso made in a specific area of Okazaki city, Aichi prefecture, Japan. Made with only soybean, salt and koji, it is aged for more than two years.
Miso (10 g) were added 30 mL of water, and the mixture was ground using a mortar and pestle. The samples were centrifuged 4000 rpm for 10 min, followed by filtrated with the filter unit (Whatman 0.45 µm, GE Healthcare UK Ltd., UK). The filtrates (400 µL) were mixed with 200 µL of the NMR cocktail [the mixture for NMR measurement, which contained D2O, 0.3 M Na-K-phosphate buffer (pH 7.4), 5 mM sodium 3-(trimethylsilyl) propionic 2,2,3,3-d4 acid (TSP) and NaN3]. The mixture was centrifuged 12,000 rpm for 5 min and the supernatant was placed in 5 mm NMR tubes (Shigemi Co. Ltd., Tokyo, Japan).
Single pulse 1H NMR spectra were recorded using 500 MHz NMR spectrometer (ECA-500, JEOL Ltd., Tokyo, Japan) equipped with the auto sample changer. Each spectrum consisted of 64 K complex data points with a spectrum width 9 kHz, where each spectrum was accumulated by 128 scans with an acquisition time of 3.49 s and a recycle delay of 5 s per scan. The water signal was suppressed by a presaturation sequence.
NMR Data Reduction and Multivariate Data AnalysisAll 1H NMR spectra were phased and baseline corrected, and the chemical shift scale was set by assigning a value of δ = 0 ppm to the signal for the internal standard TSP by ALICE2 for Windows software Version 4.1 (JEOL, Tokyo, Japan) or Chenomx NMR suite version 8.6 (Chenomx Inc., Calgary, Canada).
For multivariate data analysis, 1H NMR spectrum of miso were segmented into 0.04 ppm width over the chemical shift range of 0.5 to 9.5 ppm (excluding the region of water resonance, from 4.2 to 5.0 ppm), and each segment was integrated. The data were converted from the Chenomx software format (*.cnx) in to Microsoft Excel format (*.csv). The resultant data sets were imported into MetaboAnalyst version 5.0 (https://www.metaboanalyst.ca/) for multivariate statistical analysis. The integrated intensities were then normalized to the total spectral area, and mean-centering was applied as a data pretreatment method before principal component analysis (PCA) and partial least-squares discriminant analysis (OPLD-DA) were performed. The OPLS-DA model had verified the robustness by using the response permutation testing (RPT).
In this study, twelve of additive-free miso were used: rice (N = 5), soybean (N = 4) and barley miso (N = 3). Many commercially available miso products have ethanol added to stop fermentation or amino acids added as a seasoning, but in order to compare the original composition of miso, we used miso without additives. Details were described in Table 1. Since there are only a few types of additive-free miso on the market, and samples from different lots of the same miso produced almost identical measurement results, only data from different miso were used for the analysis. Therefore, the number of samples in this study was the minimum required for statistical analysis. Because of the small number of samples, this study will be a pilot study to investigate the characteristic components of different types of miso.
1H NMR spectra of the aqueous extract of miso were measured and applied to metabolomic analysis as described in Materials and Methods.
A representative 1H NMR spectrum of miso sample after phase and baseline correction was shown in Fig. 1. The 23 metabolites were identified and assigned using the Chenomx NMR suite version 8.6 and the results were described. In the 1H NMR spectrum, amino acids (leucine, isoleucine, valine, alanine, glutamate, glutamine, aspartate and lysine) and organic acids (lactate and acetate) were detected at 1-3 ppm, mono/poly-saccharides (glucose, maltose and glycerol) were detected at 3–5 ppm, and aromatic amino acids (tyrosine, tryptophan and phenylalanine), nucleic acids (adenine and uridine) and formate were detected at 6–9 ppm.
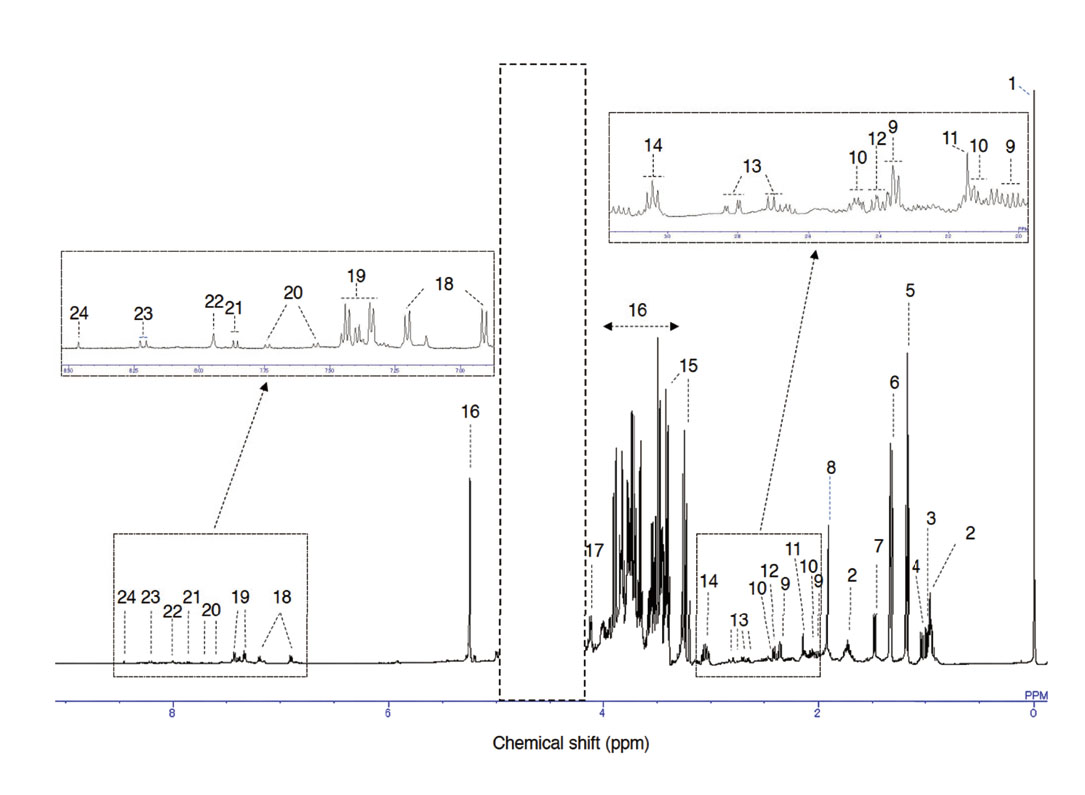
Typical 1D 1H NMR Spectrum of Miso Sample (Rice Miso)
The region of water (4.2-5.0 ppm) indicated by the dashed box was removed. The areas of 2.0-3.2 ppm and 6.9-8.5 ppm have been expanded. The following 23 metabolites (ppm) and a reference chemical (TSP) were predicted from the specific chemical shifts. 1. TSP (0.0), 2. leucine (0.9, 1.7), 3. isoleucine (1.0), 4. valine (1.0), 5. ethanol (1.2), 6. lactate (1.3), 7. alanine (1.5), 8. acetate (1.9), 9. glutamate (2.0, 2.1, 2.3), 10. glutamine (2.1, 2.4, 2.5), 11. methionine (2.1), 12. pyroglutamate (2.4, 2.5), 13. aspartate (2.7, 2.8), 14. lysine (3.0), 15. glycerol (3.6, 3.8), 16. Glucose (multi, 5.2), 17. proline (4.1), 18. tyrosine (6.9, 7.2), 19. phenylalanine (7.3, 7.4), 20. tryptophan (7.5, 7.7), 21. uridine (7.8), 22. xanthine (7.9), 23. adenine (8.2), 24. formate (8.4).
The 1H NMR spectra showed the component profiles of the miso. The integrated 1H NMR spectra of miso samples were binned to a width of 0.04 ppm, and each spectrum was converted into 206 data sets. As described in Materials and Methods, twelve 1H NMR spectra data of miso, rice miso (N = 5), soybean miso (N = 4), and barley miso (N = 3) were loaded into MetaboAnalysis 5.0, and the compositions of rice, barley, and soybean miso were compared. For an overview of the data set obtained from the 1H NMR spectra of miso samples, they were normalization by the sum and mean-centered, and then analyzed by principal component analysis (PCA). PCA was a useful tool to extract and display systematic variations in the data matrix and to identify trends and clusters in an unsupervised manner. The obtained score plot was shown in Fig. 2. From the score plot, two clearly separated clusters were identified by PCA; 95.9% of total variance was explained by two principal components (PCs), with the first principal component (PC1) accounting for 74.7% of the total variance, the second principal component (PC2) accounting for 21.22%. In PC1 of the horizontal axis, rice and barley miso were separated from soybean miso, and in PC2, segregation within rice miso was observed (Fig. 2A). The loading plot (Fig. 2 B) showed that acetate (1.94 ppm) and glucose (3.26–5.26 ppm), and amino acids such as valine (0.98 ppm) and alanine (1.50 ppm) contributed significantly to the separation of PC1. In PC2, ethanol (1.18, 3.66, and 1.22 ppm) and glucose had higher contribution.
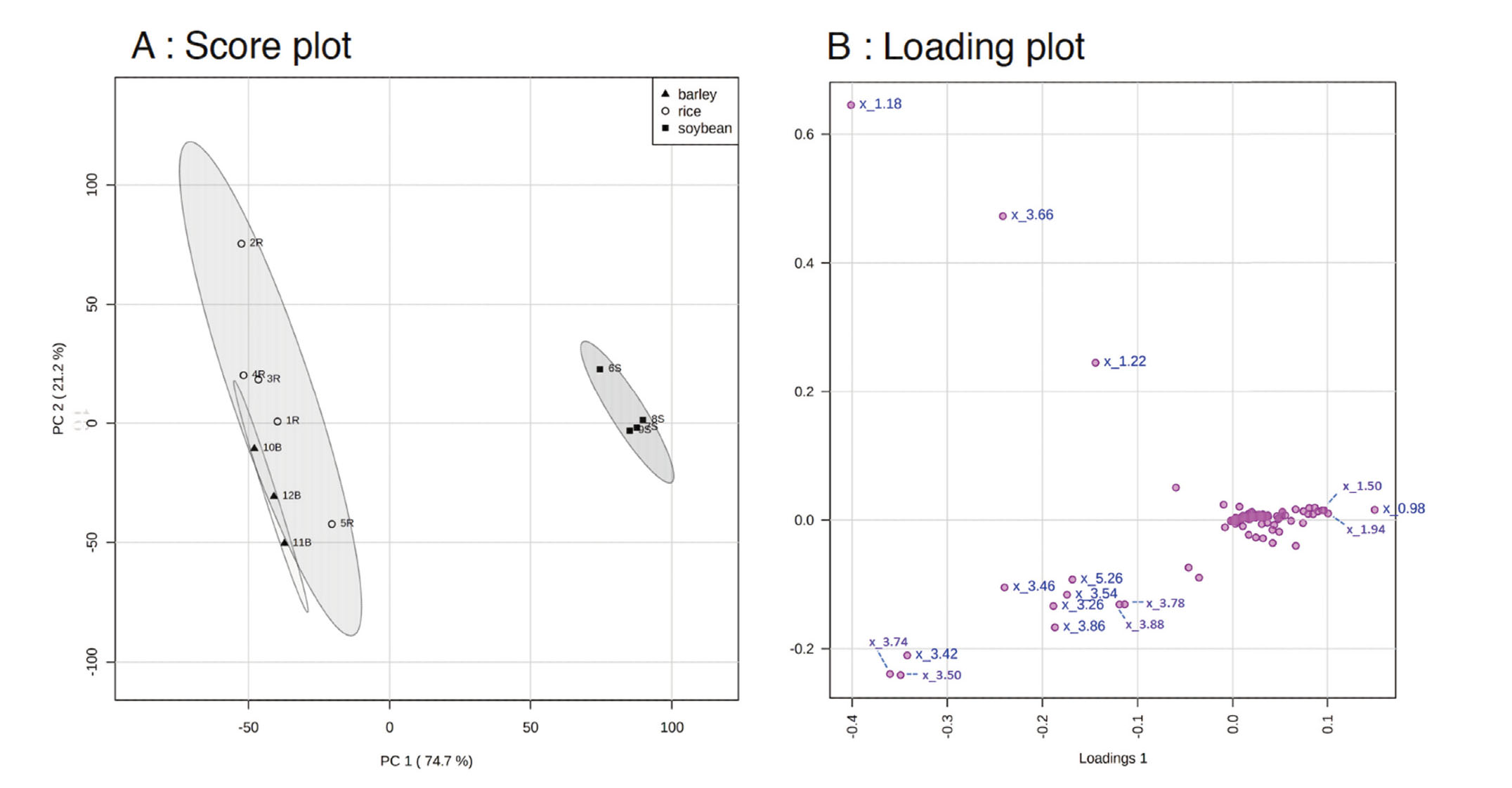
PCA Score Plot (A) and Loading Plot (B) Derived from the 1H NMR Spectra of Soybean Miso (■), Rice Miso (○) and Barley Miso (▲)
In the loading plot (B), the chemical shift (ppm) of the data that contributed to the separation of PCA were described. The dashed ellipse in score plot (A) represented the 95% confidence region.
The PCA model adequately summarized the information in the dataset and revealed differences between miso due to ingredient profiles, but the separation between rice miso and barley miso was not clear in PCA. Therefore, we performed Orthogonal Projection to Latent Structure Discriminant Analysis (OPLS-DA) for 1H NMR data sets from the three different types of miso. The OPLS-DA was a multivariate analysis method to visualize data so that group information could be used to discriminate between groups. From the OPLA-DA score plot, besides the separation of soybean miso from rice and barley miso, the separation of rice miso from barley miso was more clearly observed than in PCA with total variance of 64.2% (Fig. 3A). The explanatory (R2Y) and predictive (Q2) degrees of the target variable by the explanatory variables, which are indicators of the reliability of the created model, were 0.796 and 0.755, respectively, and a good discriminant model was obtained.
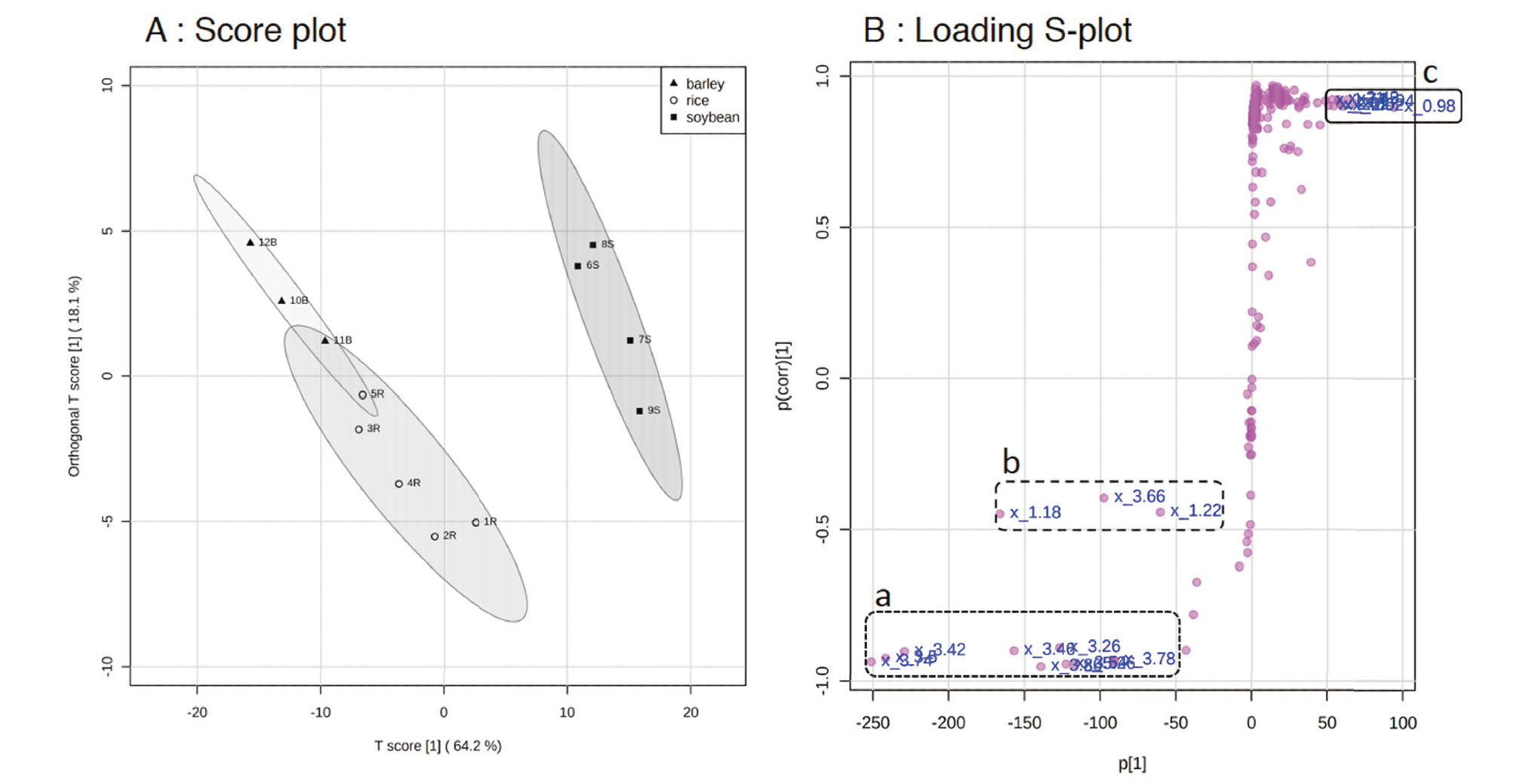
OPLA-DA Score Plot (A) and Loading S-plot (B) Derived from the 1H NMR Spectra of Soybean Miso (■), Rice Miso (○) and Barley Miso (▲)
The dashed ellipse in score plot (A) represented the 95% confidence region. The dashed boxes a, b, and c indicated the potential components identified by S-plots (B).
Potential components segregated by miso type were identified using S-plots, which are scatter plots of correlation coefficients (p corr) and covariances (p) (Fig. 3 B). The S-plots analysis revealed that metabolites in the parts a-c contribute to the differences among the three types of miso. The values for covariance (p) and correlation (p corr) of the variables in S-plots that were related to the separation of the three types of miso were shown in Table 2. |(p)| > 50 were selected. The representative components analyzed as S-plots variables were leucine/isoleucine/valine (0.94, 0.98, and 1.74 ppm), acetic acid (1.94 ppm), glutamic acid/glutamine (2.02, 2.06, 2.38, and 2.42 ppm) in part c, ethanol (1.18, 1. 22, and 3.66 ppm) in part b, and glucose/glycerol (3.04–3.74, and 5.26 ppm) in part a (Fig. 3 B).
| a | |||
|---|---|---|---|
| ppm | Estimated metabolite | p | p (corr) |
| 3.74 | glucose/glycerol | −251.2 | −0.937 |
| 3.50 | −241.8 | −0.925 | |
| 3.42 | −229.4 | −0.902 | |
| 3.46 | −157.6 | −0.900 | |
| 3.86 | −139.4 | −0.953 | |
| 3.26 | −127.0 | −0.890 | |
| 3.54 | −122.9 | −0.944 | |
| 5.26 | −117.3 | −0.947 | |
| 3.88 | −91.6 | −0.938 | |
| 3.78 | −90.9 | −0.931 | |
| b | |||
| ppm | Estimated metabolite | p | p (corr) |
| 1.18 | ethanol | −166.4 | −0.448 |
| 3.66 | −97.7 | −0.395 | |
| 1.22 | −60.5 | −0.442 | |
| c | |||
| ppm | Estimated metabolite | p | p (corr) |
| 0.98 | Val | 94.2 | 0.894 |
| 1.94 | acetate | 67.1 | 0.912 |
| 1.50 | Ala | 63.1 | 0.922 |
| 1.02 | Val/Leu/Ile | 59.9 | 0.899 |
| 2.38 | Glu | 57.8 | 0.910 |
| 2.42 | Gln | 56.9 | 0.922 |
| 2.06 | Gln | 53.9 | 0.900 |
| 0.94 | Leu/Ile | 52.9 | 0.922 |
| 2.02 | Glu | 50.7 | 0.902 |
The integration intensities associated with these component variables were shown in Fig. 4. The integration intensities of glucose (Fig. 4; A, B and C) was high in barley and rice miso, and low in soybean miso. Ethanol (Fig. 4; D, E and F) was also high in rice miso and almost undetectable in soybean miso. Among the three chemical shifts of ethanol (Fig. 4; 1.18, 1.22, and 3.66 ppm), the large dispersion at 3.66 ppm may be due to its overlap with the large spectrum of glucose. The components of rice and barley are mostly carbohydrates, and contain about 6–8% protein. One of the characteristics of barley is that it contains about 10% plant fiber, which is known to be less fermentable than rice. Therefore, it was possible that the production of ethanol by yeast was higher in rice miso.
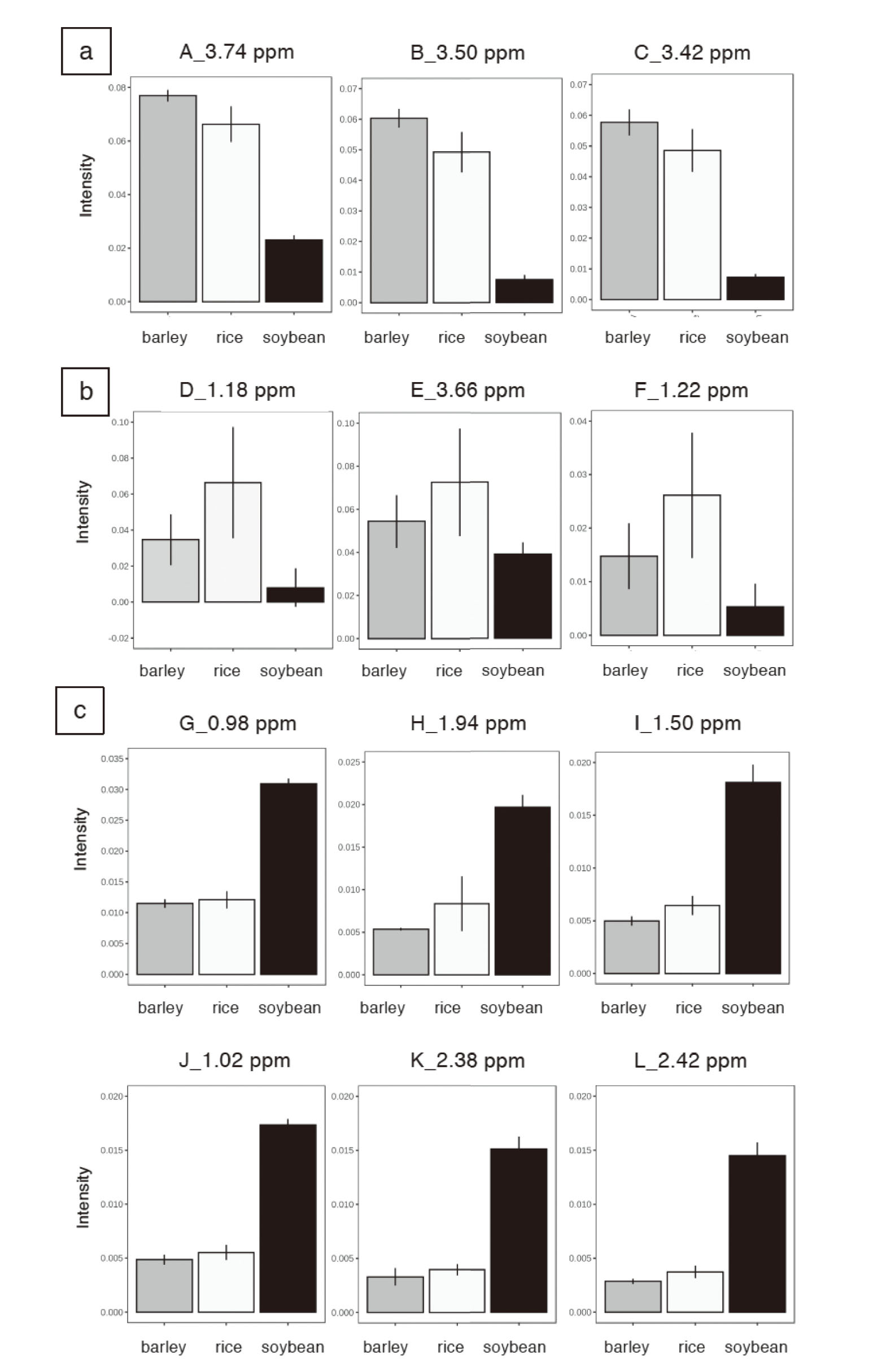
Intensity of Variables that Contributed to the Separation in the OPLS-DA Analysis
Intensities of 1H NMR spectra of variables identified from the a, b and c parts of the S-plots were compared. Gray bars represent barley miso, white bars represent rice miso, and black bars represent soybean miso. Mean ± S.D.
Compared to rice and barley miso, soybean miso contained more branched-chain amino acids such as leucine, isoleucine, and valine (Fig. 4; G–J) as well as amino acids such as glutamic acid/glutamine (Fig. 4; K and L). Rice and barley miso are also made from soybeans, but not as many amino acids have been detected as in soybean miso. The reason for this may be the difference in fermentation period. Rice and barley miso are usually consumed within a short fermentation period of about 3 months to 1 year.1,2) In the early stages of fermentation, the starch in rice and barley is digested by the koji mold (A. oryzae) to produce maltose and glucose. As a result, rice miso and barley miso have a strong sweetness. In addition, as fermentation progresses, various microflora such as yeast and lactic acid bacteria are formed, and various metabolites including lactic acid and acetic acid are produced.22) The longer the fermentation period, the darker the color of the miso becomes due to the Maillard reaction, which combines sugars and amino acids. It has been reported that the melanoidin produced by the Maillard reaction has antioxidant and active oxygen scavenging properties in miso.23) Compared to rice and barley miso, soybean miso fermented for a longer period of time, from one to four years. It is known that about 30% of soybean components are proteins, of which 10–20% are branched-chain amino acids.24) The reason why soybean miso was characterized by high levels of leucine and other branched-chain amino acids is thought to be due to the digestion of soybean protein through long-term fermentation. The carbohydrates in soybeans are also first digested into glucose, but the long-term fermentation further digested the glucose, which may be the reason that more acetic acid and formic acid were detected in the soybean miso than in the other two. As mentioned above, the characteristic components that differ among rice, barley, and soybean miso are thought to be caused mainly by differences in the raw materials (rice, barley, and soybeans) and the degree of fermentation.
Isoflavones such as genistein and daidzein, which exhibit estrogenic effects, are known to be active ingredients in foods containing soybeans,25) but their contents in miso were very low at about 50–200 μg/g,26) and they could not be detected in this study. The detection sensitivity of NMR can be improved several times by using cryogenic probes, but isoflavones cannot be detected unless they are extracted and concentrated. In addition, melanoidin, which exhibits antioxidant activity, could not be detected because it is not a single substance.
In conclusion, in this study, we used 1H NMR metabolomic analysis to compare the composition of different types of miso, a traditional Japanese fermented food. Miso can be classified into three major categories according to the ingredients. Rice miso, barley miso, and soybean miso. In this study, to investigate the characteristics of the components of commercially available additive-free rice miso, barley miso and soybean miso, we measured 1H NMR spectra of the extracts of miso and compared them using multivariate analysis. Twenty three components in miso were detected and identified by 1H NMR (Fig. 1). PCA analysis of the data set with binning of spectral integrations showed dissociation in soybean, rice, and barley miso (Fig. 2). Furthermore, OPLS-DA analysis including group information showed that barley miso and rice miso also dissociated slightly, and loading S-plots showed that amino acids including branched-chain amino acids such as leucine, isoleucine and valine were abundant in soybean miso, glucose was abundant in barley miso, and ethanol was characteristically detected in rice miso (Fig. 3, 4). Although the number of metabolites that can be analyzed by 1H NMR is smaller than that of LC/MS and GC/MS, it is easy to measure and reproducible, and can be applied to the comparison of miso components.
The measurement of 1H NMR was performed using ECA-500 at Natural Science Center for Basic Research and Development of Hiroshima University.
Conflict of interestThe authors declare no conflict of interest.