2021 Volume 4 Issue 5 Pages 155-161
2021 Volume 4 Issue 5 Pages 155-161
N-methyl-D-aspartate (NMDA) receptor has important role in synapse function and neurotransmission by the high permeability of Ca2+. In Alzheimer’s disease (AD), NMDA mediated neurotransmission is impaired by decreasing NMDA receptor subunits and increasing glutamate, which is an NMDA receptor ligand. Therefore, the NMDA antagonist (memantine) is used as a therapeutic drug of AD. Huperzia serrata is a traditional Chinese herbal medicine, and the constitute huperzine A has been reported to inhibit NMDA receptor. In this study, we clarified the effect of Huperzia serrata and the constitute huperzine A on MK-801-induced cognitive dysfunction. Memantine, Huperzia serrata and huperzine A were administered orally once a day and Y-maze test was performed at day 7 to investigate cognitive function. After Y-maze test, mice brains were collected and evaluated the expression levels of glutamate receptors and Ca2+ signalling associated protein. Memantine (5 mg/kg, p.o.), Huperzia serrata (1000 mg/kg, p.o.) and huperzine A (0.7 mg/kg, p.o.) were improved collect alternation behaviour in Y-maze test. Treatment of memantine, Huperzia serrata and huperzine A also improved the total arm entries increased by MK-801. The expression level of NMDA receptor subunit NR2A was increased by Huperzia serrata and huperzine A treatment. In addition, the decreased expression level of PKCα and phosphorylation of Erk1/2 by MK-801 were improved. These results suggest that Huperzia serrata and the constitute huperzine A improve cognitive dysfunction through NMDA receptor function and glutaminergic signaling pathway.
Glutamate is one of the most abundant amino acids in the central nervous system and plays an important role in learning and memory formation as a neurotransmitter. However, in the presence of excess glutamate, it exhibits a neurotoxic effect due to excitotoxicity.1) Glutamate works excitatory synaptic neurotransmission via ionotropic glutamate receptors. There are three ionotropic glutamate receptors, N-Methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPA), and kainate receptors.2) Synaptic dysfunction, cognitive dysfunction, and excitotoxicity caused in the presence of excess glutamate are thought to be due to altered responsiveness to Ca2+.3,4) NMDA receptor is highly permeable to Ca2+ among the ionotropic glutamate receptors. Therefore, it is suggested that the damage caused by excess glutamate is mainly mediated by the NMDA receptor.5)
In addition to higher permeability of Ca2+, NMDA receptors are characterized by voltage-gated activation by removal of Mg2+ blockade and slow ligand-gated kinetics and necessary to regulate synaptic function and plasticity.6) Synaptic plasticity changes in synaptic transmission efficiency by induction of long-term potentiation (LTP) and long-term depression (LTD). These phenomena correlate with learning such as fear conditioning and odor learning.7,8) In addition, LTP increases NMDA receptor expression, and deficiency of the NMDA receptor subunit reduces LTP and learning memory.9,10) Therefore, it is suggested that NMDA receptors are essential for cognitive function.
It has been reported that the concentration of glutamate in cerebrospinal fluid has increased in the Clinical Dementia Rating (CDR) 0.5. CDR 0.5 corresponds to mild cognitive impairment (MCI).11) In addition, impaired neurotransmission by NMDA and decreased NMDA receptor subunits have been reported in Alzheimer’s disease (AD) patients.12,13) Therefore, in the prevention of dementia, an approach to the NMDA receptor that affects cognitive function by glutamate signaling is also considered to be effective.
MK-801 is a non-competitive NMDA receptor inhibitor and has a high affinity for NMDA receptors. It is known that high-affinity NMDA receptor inhibitors such as MK-801 have a protective effect on neuronal cells. On the other hand, MK-801 excessively suppresses glutaminergic neuronal activity and inhibits LTP induction, synaptic plasticity, and learning memory formation.14) Behavioral abnormality is induced by MK-801, and MK-801 is used as an animal model for cognitive impairment associated with dementia and schizophrenia.15) In addition, MK-801 enhances glutamate release by inhibiting NMDA receptors.16,17) MK-801 is considered to induce cognitive dysfunction by disturbing glutaminergic neurotransmission.
Huperzia serrata is known as Chinese club moss and has been used in traditional Chinese herbal medicine to treat contusions, schizophrenia, and swelling.18) Recently, we reported that the improvement effect of Huperzia serrata, which is a kind of natural source of huperzine A, against cognitive dysfunction through cholinergic system.19) It has been reported that huperzine A has an NMDA receptor inhibitory effect in addition to the AChE inhibitory effect.20,21) Huperzine A has been reported to improve pentylenetetrazol, NMDA-induced seizures, kainic acid-induced seizures.22–24) However, there are no reports NMDA receptor mediated effect of huperzine A against cognitive function. In this paper, we focused on the effect of Huperzia serrata and its constituent huperzine A on MK-801-induced cognitive dysfunction and changes in the glutaminergic signaling pathway.
Six weeks old male ICR mice (Japan SLC, Hamamatsu, Japan) were used in this study. The mice were housed at 24 ± 2ºC under a 12 h/12 h light/dark cycle (lights on 8:00 to 20:00) and had ad libitum access to food and water. All procedures related to animal care and treatment conformed to the animal care guidelines issued by the Gifu Pharmaceutical University Animal Experiment Committee.
Drugs TreatmentMemantine hydrochloride (R&D Systems, Minneapolis, MN, USA) (5 mg/kg), Huperzia serrata extract (300, 1000 mg/kg) and (-)-huperzine A (Tokyo Chemical Industry, Tokyo, Japan) (0.2, 0.7 mg/kg) were dissolved in distilled water and administered orally (10 mL/kg) once a day. Huperzia serrata extract (Lot. 180328) used same sample previously extracted.25)
Y-maze TestOn day 7 of drug administration, the Y-maze test was performed to evaluate short-term and working memory. The Y-maze apparatus (40 cm in length, 10 cm in width, and 12 cm in height) consisted of three arms. Mice were treated with drug one hour before the behavioral test. Thirty minutes after drug treatment and 30 min before the behavioral test, cognitive impairment was induced by administering MK-801 (Sigma Aldrich, St. Louis, MO, USA) (0.2 mg/kg, i.p.) Saline (10 mL/kg) was administered with instead of MK-801 as the control group. At the beginning of the behavioral test, mice were placed in one fixed arm, and the number of arm alternation was observed. A correct alternation was defined as entrance into each of the three arms consecutively. Mouse behavior was recorded using a video camera, and alternations were calculated according to the following mathematical formula: Alternation (%) = ([The number of alternations]/ [Total arm invasion – 2]) × 100. The Y-maze apparatus were cleaned with 70% ethanol after every test to remove odors and excrement. Immediately after Y-maze test, mice were sacrificed, and the cortex were collected for western blot analysis.
Western Blot AnalysisMice cortex were lysed using a RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 1% Igepal CA-630] containing protease (P8340; Sigma Aldrich) and phosphatase inhibitor cocktails (P2850 and P5726; Sigma Aldrich). Lysates were centrifuged at 12,000×g for 20 min at 4°C. Protein concentrations were measured by comparison with a known concentration of bovine serum albumin, using a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Then, SDS sample buffer (Fujifilm Wako Chemicals, Osaka, Japan) were added and boiled for 5 min. Prepared samples were subjected to 5–20% SDS-polyacrylamide gel electrophoresis. The separated proteins were then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, MA, USA). The membrane were incubated with the following primary antibodies: NR2A rabbit polyclonal antibody (Upstate Biotechnology, Lake Placid, NY, USA) (diluted 1:1000), GluR1 rabbit polyclonal antibody (Cell Signaling) (diluted 1:1000), PKCα mouse monoclonal antibody (abcam, Dallas, TX, USA) (diluted 1:1000), phosphorylated ERK1/2 rabbit polyclonal antibody (Cell Signaling) (diluted 1:1000), ERK1/2 rabbit polyclonal antibody (Cell Signaling) (diluted 1:1000), and β-actin mouse monoclonal antibody (Sigma Aldrich) (diluted 1:5000). After incubation, the membrane was incubated with the following secondary antibodies: HRP-conjugated goat anti-rabbit IgG (Pierce Biotechnology, Rockford, IL, USA) (diluted 1:1000) and HRP-conjugated goat anti-mouse IgG (Pierce Biotechnology) (diluted 1:1000). The immunoreactive bands were visualized using Immunostar-LD (Fujifilm Wako Chemicals) and a Amersham Imager 680 (Cytiva, Tokyo, Japan).
Statistical AnalysisData are presented as means ± SEM. Statistical comparisons were made using Student's t-test or one-way ANOVA followed by Dunnett's test. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics (IBM, Armonk, NY, USA) software.
In Y-maze test, we examined the effect of memantine, Huperzia serrata and huperzine A on short-term and working memory. MK-801 treated mice decreased numbers of correct alternations and increased total arm entries compared with saline treated mice. Memantine (5 mg/kg) treatment significantly improved the behavioral effects induced by MK-801 (Fig. 1B, C). Similarly, Huperzia serrata (1,000 mg/kg) and huperzine A (0.7 mg/kg) treatment significantly improved correct alternations and total arm entries. Huperzia serrata (300 mg/kg) and huperzine A (0.2 mg/kg) only decreased total arm entries against MK-801 (Fig. 1D, E).
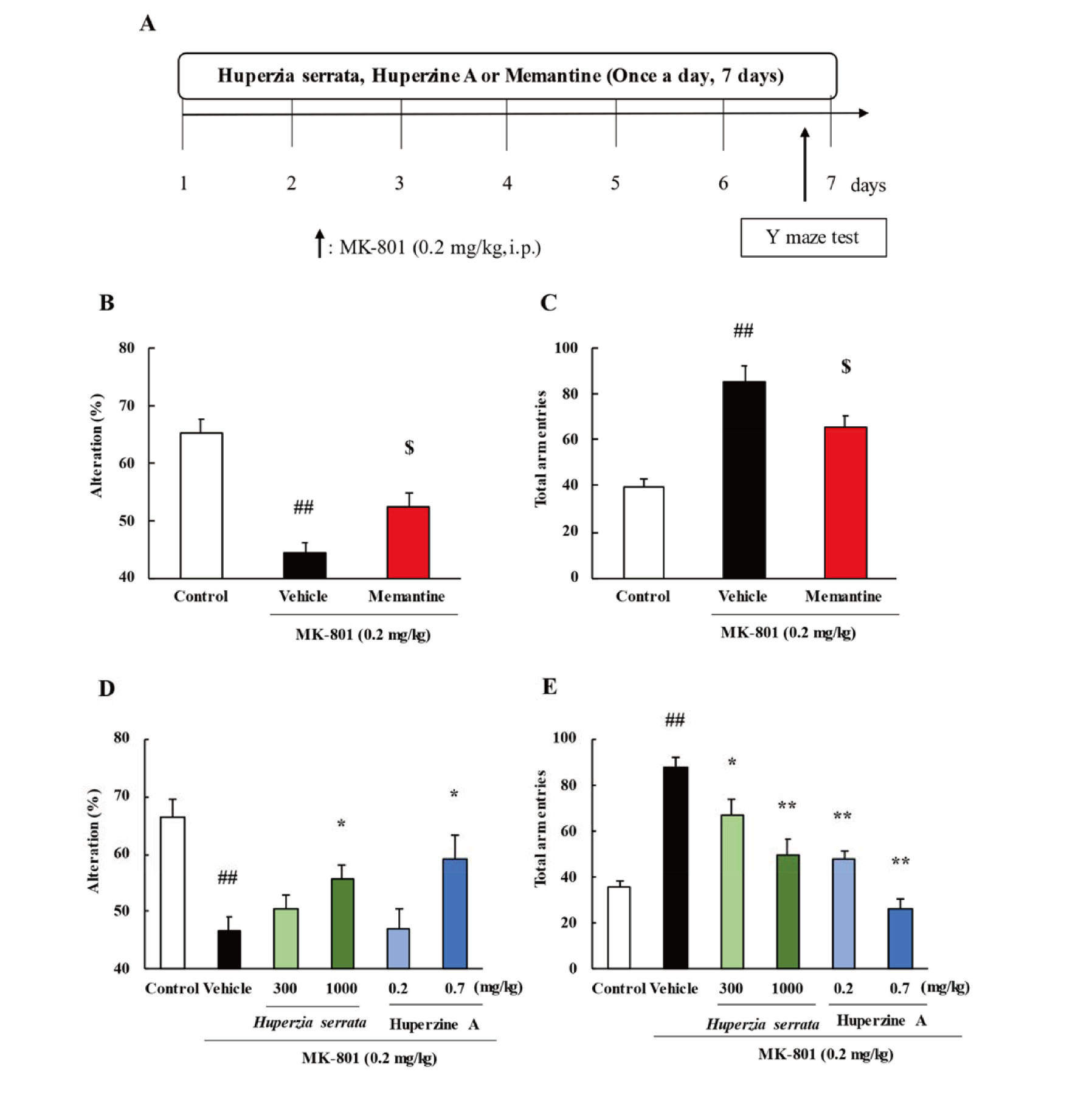
Effects of Memantine, Huperzia Serrata, and Huperzine A on Cognitive Function in MK-801 Induced Cognitive Dysfunction Mice Using Y-maze test
(A) Memantine (5 mg/kg/d), Huperzia serrata (300, 1000 mg/kg/d) or huperzine A (0.2, 0.7 mg/kg/d) was administered orally once a day for 7 d. On day 7, the Y-maze test was performed. Effects of memantine, Huperzia Serrata, and huperzine A on (B), (D) short-term memory and (C), (E) locomotor activity in MK-801 induced cognitive dysfunction mice. ##, P < 0.01 versus Control; $, P < 0.05 versus Vehicle (Student’s t-test). *, P < 0.05; **, P < 0.01 versus Vehicle (Dunnett's test). Each column and bar represent the mean ± S.E.M. (n = 7–12).
We supposed Huperzia serrata and huperzine A improved cognitive dysfunction induced by MK-801 through glutaminergic signaling pathway. Firstly, we examined the expression level of glutaminergic receptor in mice cortex. MK-801 did not affect NR1, NR2A (NMDA receptor subunit) and GluR1 (AMPA receptor subunit) expression level. Huperzia serrata (1,000 mg/kg) and huperzine A (0.7 mg/kg) significantly increased NR2A expression level compared with MK-801 treatment. NR1 and GluR1 expression level did not change by Huperzia serrata and huperzine A (Fig. 2).

The Effects of Huperzina serrata and Huperzine A on Expression Levels of Glutaminergic Receptors
(A), (C), (E) The expression levels of NR1, NR2A, GluR1 and β-actin were evaluated in the western blot analysis of mice cortex after behavioral test. The expression levels of (A) NR1, (D) NR2A and (F) GluR 1 were expressed by normalizing against β-actin. *, P < 0.05; **, P < 0.01 versus Vehicle (Dunnett’s test). Each column and bar represent the mean ± S.E.M. (n = 4–6).
Ca2+ works as a second messenger and transmits glutaminergic neural activity by intracellular influx. Protein kinase C (PKC)-MAPK pathway is a kind of pathway activated by Ca2+. We examined the expression level of PKC-MAPK pathway as a mechanism of Huperzia serrata and huperzine A. The expression level of PKCα, a member of the PKC family, and the phosphorylation of Extracellular Signal-regulated Kinase 1/2 (ERK1/2) were significantly decreased by MK-801 (0.2 mg/kg) treatment. Huperzia serrata (1,000 mg/kg) and huperzine A (0.7 mg/kg) significantly improved PKCα expression level and ERK1/2 phosphorylation (Fig. 3).

The Effects of Huperzina serrata and Huperzine A on Expression Levels of Glutaminergic Signaling
(A), (C) The expression levels of PKCα, pErk1/2, tErk1/2, and β-actin were evaluated in the western blot analysis of mice cortex after behavioral test. The expression levels of (B) PKCα was expressed by normalizing against β-actin. The phosphorylation levels of (D) ERK1/2, are also shown. #, P < 0.05; ##, P < 0.01 versus Control (Student’s t-test). *, P < 0.05; **, P < 0.01 versus vehicle (Dunnett’s test). Each column and bar represent the mean ± S.E.M. (n = 4–6).
In this paper, we examined the effects of Huperzia serrata and huperzine A on cognitive impairment through the NMDA receptor. Both memantine and MK-801 are non-competitive NMDA receptor inhibitors and have been reported to have neuroprotective effects on ischemia and oxidative stress.26,27) On the other hand, MK-801-induced cognitive dysfunction in normal rats differ from memantine.28) In addition, MK-801 has a glutamate release enhancing effect. In this paper, we used MK-801-induced cognitive dysfunction model to mimic the glutamate excess state in AD. Cognitive dysfunction induced by MK-801 was improved by administration of memantine, an AD therapeutic drug. It has been reported that memantine restores the NMDA receptor current by MK-801.29) It is suggested that memantine improved cognitive dysfunction by normalizing Ca2+ influx and glutamate signaling. Memantine binds to the MK-801 binding site of the NMDA receptor. Memantine dissociates from NMDA receptor when a signal for memory formation enters and transmits the signal. On the other hand, MK-801 does not dissociate from the receptor even when a signal for memory formation is input. Therefore, it is considered that cognitive dysfunction was suppressed by inhibiting the binding of MK-801 to NMDA receptor. Excessive release of glutamate and dopamine has also been shown to increase activity,30,31) and MK-801 increased total arm entries in the Y-maze test. Memantine and MK-801 have similar binding sites for NMDA receptors. Therefore, it is suggested that memantine suppressed the excessive glutamate release by MK-801 and reduced total arm entries. From these results, MK-801-induced cognitive disfunction is a useful animal model to examine the effect of NMDA receptor inhibitors on cognitive function such as memantine.
Huperzia serrata (1000 mg/kg, p.o.) and huperzine A (0.7 mg/kg, p.o.) improved cognitive dysfunction induced by MK-801 in Y-maze test. The huperzine A content in the Huperzia serrata extract used in this paper was 0.069%.25) Therefore, 1000 mg/kg of Huperzia serrata is equivalent to about 0.7 mg/kg of huperzine A. Huperzia serrata (1000 mg/kg, p.o.) and huperzine A (0.7 mg/kg, p.o.) also decreased total arm entries in Y-maze test. The effects of Huperzia serrata and huperzine A against MK-801-induced cognitive dysfunction is related to the NMDA receptor inhibitory action. Huperzine A also binds to the MK-801 binding site of the NMDA receptor. Therefore, like memantine, when a signal for memory formation is input, it is considered to dissociate from the receptor and transmit the signal. Therefore, it is considered that huperzine A also suppressed cognitive dysfunction by inhibiting the binding of MK-801. It has been reported that (-)-huperzine A has a stronger inhibitory effect on AChE than (+)-huperzine A, but the inhibitory activity on NMDA receptors is similarly.20) Therefore, it is suggested that there is no difference in the effects of (-)-huperzine A and (+)-huperzine A on MK-801-induced cognitive dysfunction. On the other hand, Huperzia serrata (300 mg/kg, p.o.) and huperzine A (0.2 mg/kg, p.o.) did not improve cognitive dysfunction but decreased in total arm entries. These results may involve the action of huperzine A on GABA receptors. Previously, it has been reported that quercetin reduced hyperactivity induced by MK-801 reducing GABAergic neurotransmission and regulating nerve excitatory-suppressive balance.32) Huperzine A suppressed pentylenetetrazol, a GABA receptor inhibitor induced spasm.23) It is suggested that huperzine A reduced MK-801-induced hyperactivity by regulating GABAergic neurotransmission.
Glutamate neurotransmission regulates neuroplasticity through neurotrophic factors and is involved in learning and memory formation.33) The NMDA receptor has NR1 subunit and NR2 subunit. NR2 subunit is further classified into NR2A, NR2B, NR2C, and NR2D subunit. Huperzia serrata (1000 mg/kg, p.o.) and huperzine A (0.7 mg/kg, p.o.) increased NR2A subunit expression in MK-801 treated mice. In addition, the administration of MK-801 did not affect the expression level of NR2A subunit. It has been suggested that decreased expression of NMDA receptors causes working memory impairment. Among them, loss of NMDA receptors including the NR2A subunit predicts the severity of working memory impairment.34) Huperzia serrata (1000 mg/kg, p.o.) and huperzine A might enhance NMDA receptor current necessary for memory formation by increase of NR2A expression. Higher concentration (1.0 mg/kg, i.p.) of MK-801 has been reported to reduce NR2A expression,35) but MK-801 (0.2 mg/kg, i.p.) did not affect NR2A subunit expression. Therefore, it is possible that MK-801 (0.2 mg/kg, i.p.) decreased the function of the NMDA receptor. Administration of MK-801 (1.0 mg/kg) was difficult to evaluate correct cognitive function due to gait abnormality of mice. In this paper, we used the dose concentration of MK-801 (0.2 mg/kg) that could induce cognitive dysfunction. On the other hand, the expression level of GluR1, one of the AMPA receptor subunits, did not change. AMPA receptors also permeate Ca2+, but Huperzia serrata and huperzine A are involved in Ca2+ influx and signal transduction via NMDA receptors.
The binding of glutamate to the NMDA receptor opens the NMDA receptor and induces the intracellular Ca2+ influx. Flowed intracellular Ca2+ acts as a second messenger and induces LTP, which is important phenomenon for learning and memory formation.5) Erk1/2 is phosphorylated during LTP induction and suppression of Erk1/2 phosphorylation inhibits LTP induction.36) In addition, the formation of learning memory is inhibited by Erk inhibitors.37) Thus, Erk phosphorylation is involved in synaptic plasticity and cognitive function by glutamate signaling. PKC is a protein kinase that is activated by Ca2+. In the hippocampus, PKC is known to regulate Erk phosphorylation via Ras/Raf/MEK/Erk pathway.38) In addition, PKC inhibitors inhibit the induction of LTP similarly Erk inhibitors.39) From these reports, it is suggested that the PKC-Erk pathway regulates cognitive function through glutamate signaling. The expression level of PKCα and phosphorylation of Erk1/2 were reduced by administration of MK-801. It is considered that MK-801 induced cognitive dysfunction was caused by decreased activation of the PKC-Erk pathway. Huperzia serrata (1000 mg/kg, p.o.) and huperzine A (0.7 mg/kg, p.o.) improved PKCα expression level and Erk1/2 phosphorylation in MK-801 administered mice. These results suggest that Huperzia serrata and huperzine A increased the function of NMDA receptors and improved intracellular PKC-Erk pathway activation. As a result, Huperzia serrata and huperzine A improved the cognitive dysfunction.
In conclusion, we clarified that Huperzia serrata and huperzine A improved MK-801-induced cognitive dysfunction, and that its action was regulation of glutamate signaling pathway mediated by NMDA receptor.
This study was supported by METI Monozukuri R&D Support Grant Program for SMEs Grant Number JPJ005698.
Conflict of interestThe authors declare no conflict of interest.