2023 Volume 6 Issue 2 Pages 43-46
2023 Volume 6 Issue 2 Pages 43-46
We have previously reported the generation of Rassf6 knockout mice using CRISPR/Cas technology. Furthermore, we have reported that RASSF6 is implicated in the regulation of the canonical nuclear factor (NF)-κB signaling pathway and that 7,12-dimethylbenz(a)anthracene induced skin inflammation in Rassf6 knockout mice more remarkably than in wild-type mice. In this study, we investigated the role of RASSF6 in skin tumorigenesis using a two-stage carcinogenesis model in Rassf6 knockout mice. Rassf6 knockout mice initially developed significantly more papillomas than did the control wild-type mice. Additionally, macrophages were detected and the canonical NF-κB signal was elevated in papillomas of Rassf6 knockout mice. These results indicated that canonical NF-κB signaling may be involved in papilloma formation. The suppression of NF-κB signaling may have implications for preventing cancer initiation and progression. The findings of this study indicate a tumor suppressive role of RASSF6.
Ras-association domain family (RASSF) comprises 10 genes in the human genome. Six of them, RASSF1–6, belong to a subgroup called C-RASSF.1) C-RASSF expression is often suppressed in human cancers, and cancers with low expression of C-RASSF become malignant and have a poor prognosis; therefore, it is considered a tumor suppressor molecule.
In a previous study, we generated Rassf6 knockout mice using the CRISPR/Cas technology to confirm that RASSF6 deficiency enhances tumor susceptibility.2) Nevertheless, Rassf6 knockout mice did not develop tumors. Moreover, Rassf6 knockout mouse embryonic fibroblasts (MEF) were found to be less prone to p53-dependent cell death and cell-cycle arrest and exhibited inhibition of cell senescence and delayed DNA damage repair. Furthermore, the introduction of activated KRAS into Rassf6-null MEF promoted colony formation, whereas the introduction of MYC, activated TAZ, and oncogenic p53 did not result in colony formation. RNA sequencing analysis showed that nuclear factor (NF)-κB signaling was enhanced in Rassf6-null MEF. Moreover, Rassf6 knockout mice exhibited increased NF-κB signaling and more remarkable skin inflammation than wild-type mice. RASSF6 deficiency might have compromised p53 function and enhanced NF-κB signaling, causing tumorigenesis.
As mentioned previously, Rassf6 knockout mice did not exhibit spontaneous tumorigenesis. However, it has been reported that mice knocked out for Rassf1a, which encodes another C-RASSF, are susceptible to spontaneous tumorigenesis and chemical-induced tumorigenesis.3) RASSF6 has also been reported to exert a tumor suppressive effect mediated by p53 and Rb in experiments using human cancer cells.4,5) Therefore, it is presumed that the susceptibility to tumorigenesis would be changed in deficient mice if tumorigenesis was promoted. In this study, we performed a two-stage carcinogenesis experiment to clarify the signaling of RASSF6 during tumorigenesis.
We used 7–9-week-old female wild-type and Rassf6 knockout mice (n = 5 per group) in the animal study.2) All animal experiments were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University (Approval #A2021-392).
In Vivo Two-Stage Skin Carcinogenesis ExperimentAfter hair shaving, the mice were painted on the dorsal skin with 100 μg of 7,12-dimethylbenz[a] anthracene (DMBA; Tokyo Chemical Industry, Co. Ltd) in 200 μL of acetone for tumor initiation. One week later, 30 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA; Nacalai Tesque, Japan) in 200 μL of acetone was applied to the same area twice a week for 18 weeks, to promote papilloma formation.6) The number of papillomas was assessed weekly. Developing papillomas were counted when they reached at least 1 mm in diameter.
Antibodies and ReagentsThe antibodies and used here reagents were obtained from the commercial sources mentioned below: rabbit anti-α-tubulin (PM054) (Medical and Biological Laboratories Co. Ltd.); rabbit anti-IκBα (4812) and rabbit anti-phospho-IκBα (2859) (Cell Signaling Technology); and rat anti-F4/80 (ab6640) (Abcam) and anti-DMBA (D0677) (Tokyo Chemical Industry, Co. Ltd.); TPA (27547-14) (Nacalai Tesque, Japan).
Western BlottingFive papillomas less than 5 mm were collected in one mouse as one sample. Three samples were created per group and used for the experiment. Papillomas were treated in RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 10 mM NaF, 1 mM APMSF). After that, they were homogenized and protein concentration determined. Moreover, they were dissolved in SDS sample buffer (0.5 M Tris/HCl, pH 6.8 62.5 mM, SDS 2%, glycerol 10%, 2-mercaptoethanol 5%, Bromophenol blue 0.02%), and boiled at 96°C for 5 min. Appropriate amounts of proteins were separated on 8%–15% acrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked with 5% (w/v) milk in TBS–Tween buffer (50 mm Tris, 138 mm NaCl, 2.7 mm KCl, 0.1% [v/v] Tween 20, pH 7.4) and incubated overnight at 4°C with the indicated antibodies. The membranes were washed in TBS–Tween buffer, incubated with the appropriate secondary antibodies for 1 h at room temperature with shaking, and washed again in TBS–Tween buffer. Signals were detected using the ECL (GE Healthcare) reagent and X-ray films. The signals were measured with ImageJ.
Statistical AnalysisFor the comparison between two samples, statistical analyses were performed using Student’s t-test with the R software, ver. 4.05 GUI 1.74.
Papillomas were initiated with a single dose of DMBA and promoted using TPA twice per week (Fig. 1a, 1b). Compared with WT mice, Rassf6 knockout mice exhibited a remarkable increase in both the incidence (Fig. 1c) and the number (Fig. 1d) of skin papillomas. The period necessary for all mice to form papillomas was 14 weeks for WT and 9 weeks for Rassf6 knockout mice. In Rassf6 knockout mice, the period until papilloma formation was shortened in all mice compared with WT mice. When examined separately according to tumor diameter at 18 weeks, both 1– < 3 mm and 3– < 5 mm tumors showed increased papilloma formation in Rassf6 knockout mice compared with WT mice. At a diameter > 5 mm, there was no papilloma formation in WT mice, whereas there was papilloma formation in Rassf6 knockout mice (Fig. 1e).
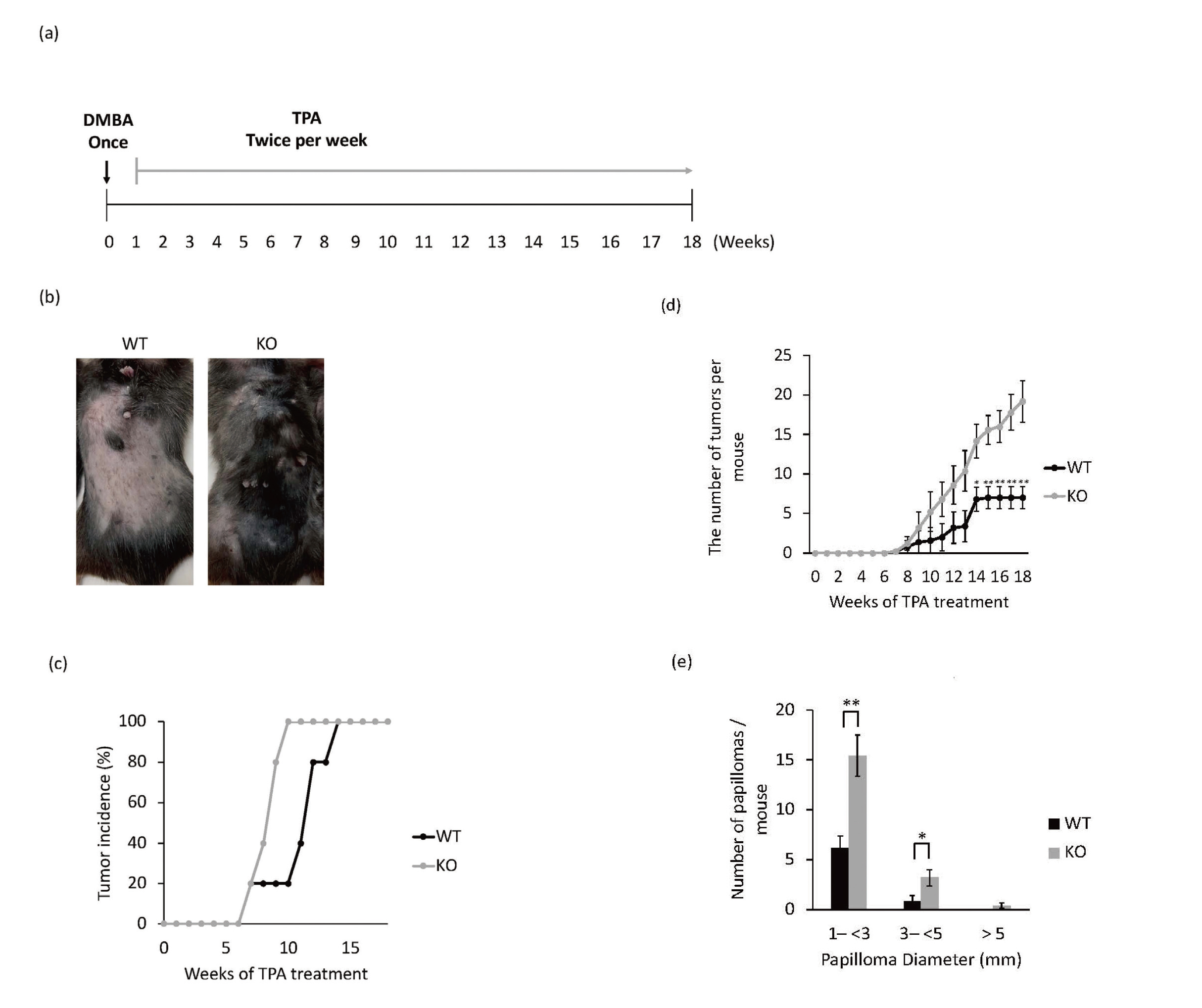
Promoted Two-Stage Skin Carcinogenesis in the Absence of RASSF6
(a) Schematic diagram of a two-stage carcinogenic experiment in the mouse skin.
(b) Formation of papillomas in DMBA/TPA-induced WT and Rassf6 knockout mice at 18 weeks.
(c) Incidence of papillomas in the different treatment groups.
(d) Number of papillomas per mouse in the indicated groups.
(e) Average numbers of papillomas per mouse in group with different tumor diameters. Data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01.
Immunoblotting with anti-macrophage F4/80 antibody revealed macrophage infiltration in papillomas of Rassf6 knockout mice (Fig. 2a).
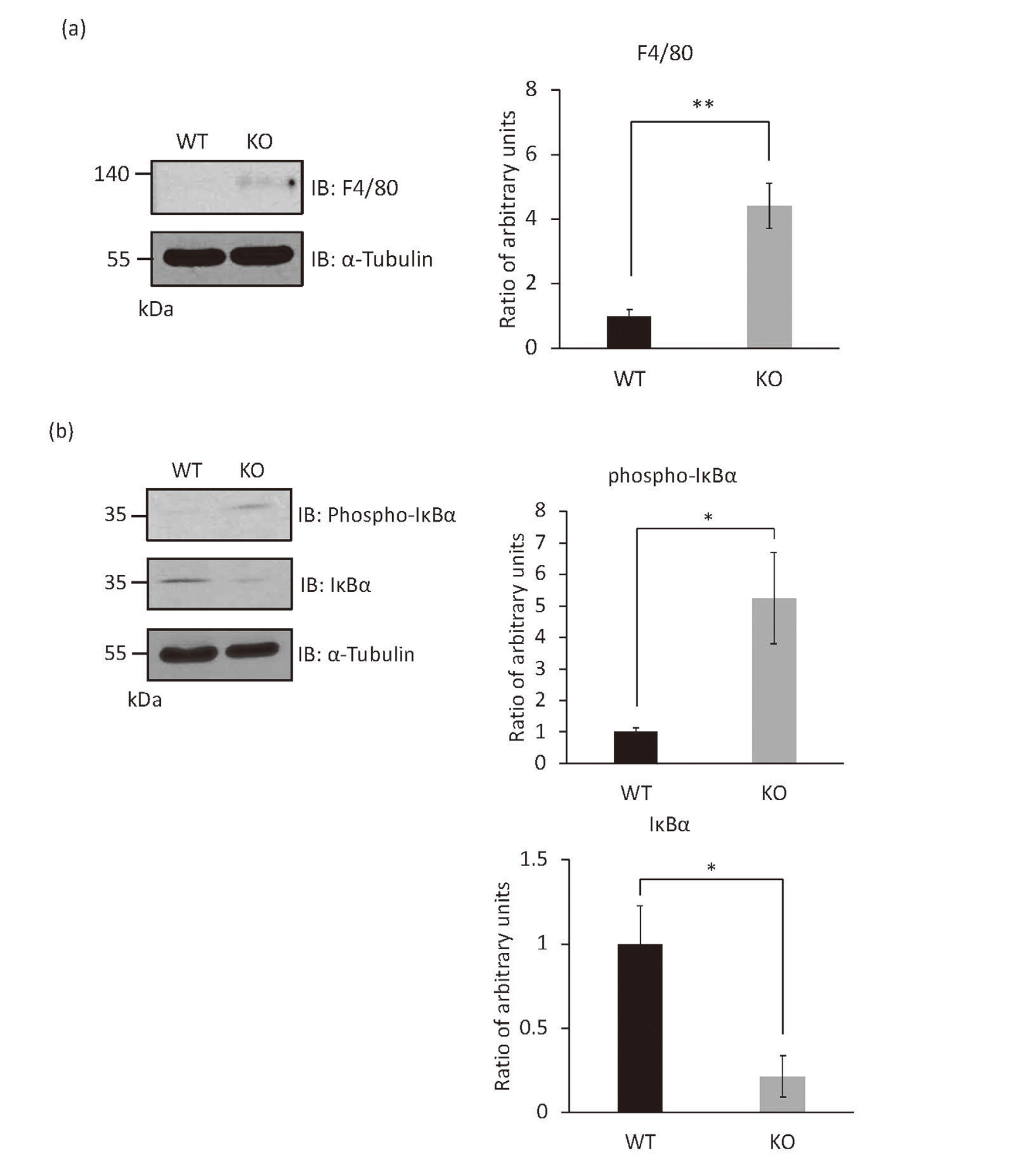
The Canonical NF-κB Signaling Pathway Is Enhanced in Papillomas of Rassf6 Knockout Mice
(a) The tissue lysates from papillomas of wild-type and Rassf6 knockout mice were immunoblotted with the indicated antibodies. Quantification graphs are shown with α-Tubulin as an endogenous control for protein expression. Protein levels are represented as mean ratio values quantified from protein bands of each marker versus α-Tubulin compared to wild-type or Rassf6 knockout mice.
(b) The tissue lysates from papillomas of wild-type and Rassf6 knockout mice were immunoblotted with the indicated antibodies. Quantification graphs are shown with α-Tubulin as an endogenous control for protein expression. Protein levels are represented as mean ratio values quantified from protein bands of each marker versus α-Tubulin compared to wild-type or Rassf6 knockout mice.
Data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01.
The immunoblotting experiment revealed the enhancement of IκBα phosphorylation and the downregulation of IκBα (Fig. 2b). Therefore, the canonical NF-κB signaling pathway seems to be enhanced in papillomas of Rassf6 knockout mice.
We reported previously the upregulation of NF-κB and the presence of hyperinflammatory responses in the skin of Rassf6 knockout mice.2) Here, we found that NF-κB was similarly upregulated in papillomas of Rassf6 knockout mice. However, no spontaneous tumorigenesis was observed, and it is conceivable that chemical stimulation promotes tumorigenesis.
In this study, we also clarified that macrophage infiltrated in papillomas of Rassf6 knockout mice. In a previous study, it reported that the Rassf6 mRNA level is inversely correlated with the number of macrophages in adipose tissues.7) The molecular mechanism via which RASSF6 suppresses NF-κB signaling warrants further investigation. The analogy with RASSF1A and RASSF2 predicts that RASSF6 binds to TBK1 and IKKα/β.8,9)
In addition, two scenarios are hypothesized from the viewpoint of the pathological significance of RASSF6-deficiency-induced NF-κB signal enhancement. First, inflammatory cytokines are secreted from cancer cells with decreased expression of RASSF6, and inflammatory cells, such as macrophages, are recruited around the cancer tissue, thus creating a favorable microenvironment for cancer growth. The second scenario is one in which decreased expression of RASSF6 is involved in the establishment of chronic inflammation, which is a major cause of tumorigenesis.
In both scenarios, suppression of NF-κB signaling may have implications for preventing cancer initiation and progression. In a previous research, canonical NF-κB signaling promotes DMBA/TPA-induced skin carcinogenesis.10) Therefore, higher canonical NF-κB signaling is predicted to promote papilloma growth. Moreover, for application in human cancers, it is necessary to investigate whether inflammatory cell infiltration is observed around cancers with low RASSF6 expression using human tissue. Furthermore, it is necessary to analyze whether RASSF6 expression is already reduced during chronic inflammation prior to tumorigenesis.
This work was supported by JST SPRING, Grant Number JPMJSP2120. We thank Dr. Yutaka Hata (Tokyo Medical and Dental University) for various materials. The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflict of interestThe authors declare no conflict of interest.