2023 Volume 6 Issue 3 Pages 103-107
2023 Volume 6 Issue 3 Pages 103-107
The epithelial–mesenchymal transition (EMT) of endometrial cells contributes to the development of endometrial cancer, endometriosis, and adenomyosis. We have recently reported that 12-O-Tetradecanoylphorbol 13-acetate (TPA), a protein kinase C activator, induces EMT in HHUA endometrial cells cultured on collagen type I gels. HHUA cells showed an obvious morphological change from spheroids to scattered spindle cells during the TPA-induced EMT. In this study, we searched for inhibitors of the TPA-induced morphological change, and isolated vitetrifolin D from Vitex rotundifolia leaves. Vitetrifolin D suppressed the TPA-induced downregulation of E-cadherin, a major component of the epithelial adherens junction. Since vitetrifolin D showed a modest cytotoxicity at the concentration required to inhibit E-cadherin downregulation, it would not be suitable as a drug to treat endometrial disorders. However, our cell morphology-based screening could contribute to the discovery of new compounds that inhibit the loss of epithelial cell junctions.
The epithelial–mesenchymal transition (EMT) is a cellular process during which epithelial cells lose cell–cell junctions, apical–basal polarity, and interactions with the basement membrane, and acquire mesenchymal phenotypes including fibroblast-like morphology and enhanced motility.1) Since EMT is an initial step in cancer progression and fibrosis, it has been an attractive target for the development of new therapeutic strategies. Specifically, EMT in the endometrium is a factor predisposing women to not only endometrial cancer but also endometriosis and adenomyosis, all of which are common and benign gynecological disorders.2,3) In these disorders, endometrial cells travel and attach to other tissues or invaginate into the myometrium, resulting in ectopic endometrium that causes bothersome symptoms including menstrual pain, abdominal pain, and abnormal uterine bleeding.4) The mesenchymal character of ectopic endometrial cells and fibrotic tissue around lesions are common pathological features of endometriosis and adenomyosis,3,5–7) suggesting that regulation of endometrial EMT is important for prevention and treatment of these diseases.
We have recently reported the unique behavior of HHUA endometrial cells cultured on collagen type I (COL-I) gels.8) HHUA cells aggregated and formed spheroids when cultured on COL-I gels. 12-O-Tetradecanoylphorbol 13-acetate (TPA), an inducer of inflammation via protein kinase C activation, disassembled the spheroids and increased the cell proliferation rate. The TPA-treated cells showed a fibroblast-like spindle shape with protrusions and reduced adhesion to each other. Moreover, TPA reduced the expression of E-cadherin, a major component of the epithelial adherens junction, and increased vimentin, an intermediate filament protein of mesenchymal cells. Since HHUA cells show an obvious morphological change, we conceived the application of this in vitro model to cell morphology-based screening to discover novel EMT inhibitors.
In this study, we searched for natural products that inhibited the TPA-induced morphological change of HHUA cells cultured on COL-I gels. We focused on Vitex rotundifolia L. f. (Lamiaceae) that is widely distributed at the Asia and Oceanian coast, since plants of the genus Vitex have been used to treat variety of diseases, including menstrual pain and premenstrual syndrome.9) We found the leaf extract suppressed the morphological change, and identified vitetrifolin D (VitD) as an active component. Western blotting analysis and WST-based cytotoxicity assay revealed that VitD inhibited TPA-induced downregulation of E-cadherin at concentrations that moderately affected cell viability.
NMR spectra were recorded on a JNM-ECZ500 and ECZ-600 (JEOL, Tokyo, Japan), and chemical shifts are reported in ppm relative to the residual solvent (CDCl3 as δ = 7.26 ppm). High-resolution electrospray ionization mass spectra (HR-ESI-qTOF-MS) were recorded on a micrOTOF II (Brucker Daltonics, Billerica, MA, USA). Wakogel C-300 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and COSMOSIL 75C18-OPN (Nacalai tesque, Inc., Kyoto, Japan) were used for column chromatography. YMC Pack SIL SL12S05-1510WT (YMC Co. Ltd., Kyoto, Japan) was used for HPLC. TPA was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Cell CultureThe human endometrial cancer cell line, HHUA (RCB0658),10) was provided by RIKEN BRC through National Bio-Resource Project of the NEXT/AMED (Tsukuba, Japan). The cells were cultured in Ham’s F-12 medium with 10% heat-inactivated FBS (Corning, NY, USA), 1 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) in a 5% CO2 humidified incubator maintained at 37°C.
Preparation of Collagen Type I Gel-Coated Plate0.2% of atelocollagen solution (3D-LG, lot. 362011, KOKEN Co., Ltd., Tokyo, Japan) was added to each well of a culture plate (150 μL/cm2) and incubated at 37°C for 2 h to allow gelation.
Cell Morphology AssayHHUA cells (1 x 104 cells/well) were seeded in a COL-I gel-coated 48-well plate and allowed to attach overnight. A sample solution of DMSO or EtOH was added. After incubation for 30 min, TPA solution in DMSO was added and incubated for another 5–24 h. These cells were observed using a phase-contrast microscope (ECLIPS Ts2; NIKON, Tokyo, Japan).
Plant MaterialThe plant Vitex rotundifolia L. f. (Lamiaceae) was collected from Chidorigahama Beach (Naruto, Japan) in December 2021. The voucher specimen was deposited at the Department of Applied Biological Science, Faculty of Agriculture, Kagawa University.
Extraction and IsolationThe leaf extract of V. rotundifolia was partitioned based on the result of cell morphology assay. A solution of each fraction was added to HHUA cells cultured on COL-I gels before TPA solution was added. After incubation for 5 h, active fractions were determined by microscopic observation and subjected to further purification as shown below.
Fresh leaves of V. rotundifolia (230 g) were extracted with methanol (800 mL) at room temperature. The methanol extract (32 g) was partitioned between n-hexane and 90% methanol. The n-hexane extract (530 mg) was subjected to silica gel column chromatography (ϕ 25 x 100 mm; 0–100% EtOAc in hexane) to give 7 fractions. The elute obtained with 40% EtOAc in n-hexane (63 mg) was subjected to ODS column chromatography (ϕ 15 x 30 mm; 50–100% MeOH in H2O) to give 6 fractions. The elute obtained with 80% MeOH in H2O (6.8 mg) was subjected to normal phase HPLC (YMC Pack SIL SL12S05-1510WT, ϕ 10 x 150 mm; 20% EtOAc in n-hexane; flow rate 3.0 mL/min) to give vitetrifolin D (0.37 mg): 1H-NMR spectrum (Supplementary material); HR-ESI-qTOF-MS m/z 429.2611 [M+Na]+ (calcd for C24H38O5Na, 429.2611).
Western BlottingHHUA cells (7.5 x 104 cells/well) were seeded in a COL-I gel-coated 48-well plate and allowed to attach overnight, and then VitD solution in EtOH or EtOH alone was added. After incubation for 30 min, TPA solution in DMSO or DMSO alone was added and incubated for another 16 h. Cells and COL-I gels were washed with PBS, lysed by adding 100 μL of 2X SDS sample buffer, and boiled for 10 min. SDS-PAGE was performed according to the method of Laemmli11) in slab gels consisting of a 10% acrylamide separation gel and a 3% stacking gel. Western blotting was performed as described previously.12) Anti-E-cadherin (24E10), anti-vimentin (D21H3), anti-GAPDH (D16H11), and HRP-conjugated anti-rabbit IgG antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). These antibodies were diluted 1:1000 and used for the detection of immunoreactive bands. The intensity of each band was quantified using Amersham Imager 680 analysis software (GE healthcare, Chicago, IL, USA).
Cytotoxicity AssayHHUA cells (1 x 104 cells/200 μL·well) were seeded in a 96-well plate. For an assay using a COL-I gel-coated plate, 50 μL of 0.2% atelocollagen solution was added to each well of a 96-well plate and incubated at 37°C for 2 h, before HHUA cells (1 x 104 cells/150 μL·well) were seeded. After overnight preincubation, a solution of VitD in EtOH or EtOH alone was added. After incubation for 30 min, TPA in DMSO or DMSO alone was added. After incubation for 24 h, 20 μL of Cell Counting Kit-8 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was added to each well, and the plate was incubated at 37°C for another 4 h. The absorbance was measured at 450 nm using a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waithman, MA, USA). The cell number in the presence of VitD and TPA was plotted as a percentage relative to the vehicle group. Statistical significance was evaluated by Dunnett’s test using R software version 4.2.0 and indicated as *, P < 0.05; and **, P < 0.01.
Although previous studies have shown that the extracts of V. rotundifolia possess various pharmacological activities,13) their effects on EMT phenotypes have not been reported. A characteristic feature of EMT is that epithelial cells change to a spindle-shaped morphology.1) We found that the methanol extract of V. rotundifolia leaves inhibited the TPA-induced morphological change of HHUA cells cultured on COL-I gels (Fig. 1). HHUA cells started to show an elongated shape 5 h after TPA was added, and the spindle-shaped cells were obviously observed after overnight treatment. The leaf extract suppressed these morphological changes throughout 24 h. To identify the active compounds, we fractionated the extract based on the results of the cell morphology assay (Fig. 2). Since some fractions showed cytotoxicity after long-time treatment, their activity to inhibit the morphological change was determined after 5 h TPA treatment. Firstly, the leaf extract was partitioned between n-hexane and 90% methanol in water. The n-hexane extract was purified by silica gel and ODS column chromatography. Following this, normal-phase HPLC separation gave vitetrifolin D (VitD) as an active compound. The predicted molecular formula C24H38O5 from HR-ESI-MS were identical to VitD. The representative peaks of 1H-NMR were in good agreement with the literature data (Table S1),14) but some peaks were difficult to discriminate due to overlapping. Indeed, chemical shifts of these overlapped peaks were not precisely determined in the previous report that isolated VitD.14) Therefore, we also compared the 1H-NMR spectrum with that of an authentic sample (Fig. S2), and confirmed that it was identical to VitD.

The Extract of Vitex Rotundifolia Leaves Inhibited the TPA-Induced Morphological Change of HHUA Cells.
HHUA cells cultured on COL-I gels were treated with the leaf extract for 30 min before being treated with TPA for (A) 5 h and (B) 24 h. These cells were observed using a phase-contrast microscope. The cells being elongated by 5 h TPA treatment were highlighted with arrows. Scale bar: 50 μm.

Purification Scheme and Structure of Vitetrifolin D
As shown in Fig. 3, VitD suppressed the TPA-induced morphological change at 9 μM, but it was necessary to confirm whether the inhibition of morphological change was associated with EMT inhibition or nonselective cytotoxicity. To investigate whether VitD inhibited TPA-induced EMT, we first analyzed the expression of E-cadherin and vimentin in HHUA cells cultured on COL-I gels by western blotting (Fig. 4). Since the cell morphological change was completed after about 16 h TPA treatment, we predicted that EMT was completed at this time point. Thus, we lysed the cells 16 h after TPA was added. TPA reduced the expression of E-cadherin and increased that of vimentin as reported previously.8) Pretreatment of VitD (3 and 9 μM) recovered E-cadherin expression, but did not suppress vimentin upregulation, suggesting that VitD specifically inhibited the loss of the cell-cell junction but not acquisition of mesenchymal characteristics. The preserved E-cadherin expression may contribute to suppression of TPA-induced morphological change, since E-cadherin degradation is a key step in loss of cell polarity.
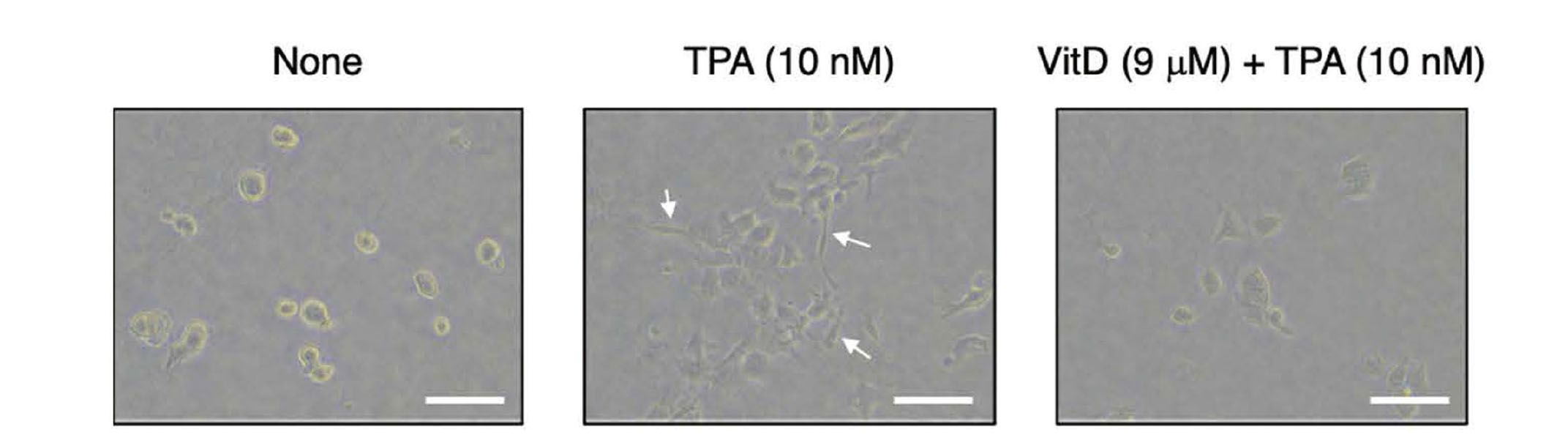
VitD Inhibited the TPA-Induced Morphological Change of HHUA Cells
HHUA cells cultured on COL-I gels were treated with VitD 30 min before being treated with TPA for 5 h. These cells were observed using a phase-contrast microscope. The cells being elongated by 5 h TPA treatment were highlighted with arrows. Scale bar: 50 μm.

VitD Suppressed TPA-Induced Downregulation of E-Cadherin
HHUA cells cultured on COL-I gels were treated with the indicated concentration of VitD before being treated with TPA for 16 h. Thereafter, cells were lysed, equal amounts of each lysate were separated by SDS-PAGE, and E-cadherin, vimentin, and GAPDH were analyzed by western blotting. Relative intensity of bands compared to vehicle control is shown above each band. A result of one of the duplicate studies that gave similar results is shown.
Next, we evaluated the effect of VitD on HHUA cell viability using a WST-based cytotoxicity assay (Fig. 5). HHUA cells were treated with VitD for 24 h, since VitD was reported to show mild cytotoxicity against pancreatic and prostate cancer cell lines after 24 h treatment.16) VitD also exhibited cytotoxicity toward HHUA cells, and the intensity was almost the same toward cells on a plastic plate as toward those on a COL-I gel-coated plate. The cell viability in the presence of 3 and 9 μM of VitD remained at 80% and 60%, respectively. As we reported previously, TPA increased the viability of HHUA cells on COL-I gels.8) VitD moderately inhibited the growth of TPA-treated HHUA cells as well as non-treated cells. Based on the results above, VitD unfortunately showed a modest cytotoxicity at concentrations that inhibited E-cadherin downregulation. However, VitD-induced suppression of E-cadherin downregulation could not be attributed merely to cytotoxicity. When we evaluated the activity of each partitioned fraction, some fractions induced cell death without suppressing the TPA-induced morphological change unlike VitD. Moreover, VitD showed a selective inhibitory activity toward E-cadherin downregulation rather than vimentin upregulation (Fig. 4). Therefore, we considered that VitD might have some specific targets that protects E-cadherin from degradation, resulting in the suppression of TPA-induced cell morphological change.

Effects of VitD on the Proliferation of HHUA Cells Cultured on (A) Plastic Plate and (B) COL-I Gel Coated Plate
HHUA cells were seeded in non-coated or COL-gel-coated 96-well plates. After overnight preincubation, cells were treated with the indicated concentration of VitD for 30 min before being added to TPA solution in DMSO or DMSO alone. After incubation for another 24 h, the cell number was estimated with the WST assay. Cell number was expressed as a percentage relative to the non-treated group. Error bars represent standard error (n = 4). * P < 0.05, ** P < 0.01 (Dunnett’s test). A result of one of the duplicate studies that gave similar results is shown.
In this study, we found that the methanol extract of V. rotundifolia leaves significantly inhibited the TPA-induced morphological change of HHUA endometrial cells. The cell morphology-guided fractionation of the extract led to the identification of VitD as an active compound. Western blotting analysis revealed that VitD suppressed the downregulation of E-cadherin, an initial step in cell invasion and metastasis, although the potency was not strong. This result suggested that our cell morphology-based assay serves as a rapid screening system to find unique natural products that inhibit the loss of epithelial adherens junctions.
VitD and related diterpenoids have been reported to show some attractive activities such as anti-tumor, anti-inflammatory, and anti-bacterial activities.15) Additionally, this study revealed a novel function of VitD as an inhibitor of E-cadherin downregulation. Arai et al. previously identified VitD as a hedgehog signaling inhibitor that disrupted the formation of the GLI1–DNA complex, resulting in growth inhibition toward some cancer cell lines.16) GLI1 is a transcription factor that is known to induce the expression of Snail, an EMT-promoting transcription factor that repressed E-cadherin expression and induced mesenchymal proteins.17) Therefore, VitD might suppress the downregulation of E-cadherin by inhibiting the GLI1–snail axis, though VitD did not prevent vimentin expression (Fig. 4). On the other hand, Tashiro et al. reported that epidermal growth factor (EGF) induced E-cadherin downregulation through the MEK/ERK pathway without any effects on the expression of mesenchymal marker proteins in LoVo colon cells.18) Since TPA can also activate the MEK/ERK pathway, 19) VitD might inhibit MEK/ERK-related proteins to suppress TPA-induced E-cadherin downregulation. We cannot immediately validate the predicted mode of actions due to the limited availability of VitD. Further study with a sufficient amount of VitD is necessary to reveal the mechanism underlying the inhibition of E-cadherin downregulation.
VitD has been isolated from V. rotundifolia, V. trifolia, V. negundo, and V. agnus-castus.15) These plants of the genus Vitex have traditionally been used for medicinal purposes. The dried fruit of V. rotundifolia and V. trifolia (“Mankeishi” in Japanese) has been used as a herbal medication for pain and inflammation relief. In addition, the fruit of V. agnus-castus (Chestberry) has been used to treat menstrual pain, dysmenorrhea, and endometriosis.20) Thus, VitD could partly contribute to suppressing these gynecological disorders by preventing loss of the cell-cell junction of endometrial epithelial cells. However, VitD may not be suitable as a new medicine, since it showed cytotoxicity at the concentration required to inhibit E-cadherin downregulation. The structure activity studies on VitD might contribute to the development of new analogs with reduced cytotoxicity.
Moreover, VitD would not be a main active compound in V. rotundifolia. Although the methanol extract of V. rotundifolia leaves inhibited the TPA-induced cell morphological change at 16 μg/mL (about 0.1 mg fresh weight equivalent extract/mL), VitD required a concentration twenty thousand times higher (9 μM, about 2 g fresh weight equivalent extract/mL) to show a similar effect (Fig. 1 and 3). Therefore, we should have missed highly active compounds that were unstable under the purification conditions or solely inactive compounds that enhanced the activity of VitD. The next step is to improve purification methods to isolate these compounds, and reevaluate their activities including the synergistic effects in combination of multiple compounds. The cell morphology assay will contribute to future studies, since it was enough sensitive to detect minor compounds such as VitD.
The authors thank Prof. Masami Ishibashi and Assist. Prof. Yasumasa Hara (Chiba University) for providing the NMR spectrum for VitD. The authors are grateful to Ms. Tsugumi Shiokawa and Dr. Hiroko Tada (Okayama University) for MS measurement. The research was supported by the Japan Society for Bioscience, Biotechnology, and Agrochemistry (the 48th Research Scholarship in 2021) and Kagawa University Research Promotion Program 2022 (Grant Number 22K0E017).
Conflict of interestThe authors declare no conflict of interest.