2023 Volume 6 Issue 3 Pages 115-121
2023 Volume 6 Issue 3 Pages 115-121
We investigated whether 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear inflammation is exacerbated in type 2 diabetic db/db mice, and if it is prevented by the control of blood glucose levels. Ear inflammation was assessed by ear thickness and the mRNA expression of the pro-inflammatory cytokines IL-1beta, IL-6, and TNF-alpha. Six-week-old db/db mice received an insulin treatment combined with exercise training daily for three weeks to lower blood glucose to normal levels. The other db/db group and their control (db/m) mice received saline without exercise training. TPA-induced ear swelling peaked after 8 h in db/m mice and after 32 h in db/db mice. The gene expression levels of cytokines 24 and 32 h after the TPA treatment were higher in db/db mice than in db/m mice. The insulin treatment combined with exercise training significantly suppressed blood glucose and HbA1c levels in 9-week-old db/db mice, but did not prevent the TPA-induced inflammatory response. These results revealed that TPA-induced ear inflammation was exacerbated in db/db mice and was not prevented by the insulin treatment combined with exercise training.
Diabetes mellitus (DM) is one of the most common diseases worldwide and its incidence is increasing.1-3) Long-term hyperglycemia may lead to several complications, including neuropathy, nephropathy, and retinopathy.2) Impaired wound healing is a frequent and severe issue in patients with DM that often results in limb loss and disability.3-5) The wound healing process comprises three overlapping phases: inflammation, proliferation, and remodeling, the dysregulation of which at each step may impair wound healing.3) We previously reported that ear inflammation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) was exacerbated in streptozotocin (STZ)-induced diabetic mice.6) This phenomenon may be similar to the dysregulation of the inflammatory phase in the wound healing process. Although impaired wound healing has been reported in STZ-induced diabetic mice and type 2 DM db/db mice,7) it currently remains unclear whether TPA-induced inflammation is exacerbated in db/db mice.
DM was recently identified as a risk factor for the more rapid progression and a worse prognosis of coronavirus disease 2019 (COVID-19).8) Although the mechanisms by which DM causes severe symptoms are unclear, the up-regulated expression of inflammatory cytokines, known as the cytokine storm, has been suggested to play a role.9) Furthermore, the DM-induced exacerbation of TPA-induced inflammation described above may partially replicate the cytokine storm. Therefore, it is clinically significant to elucidate the mechanisms underlying the DM-induced exacerbation of inflammation and develop preventative strategies.
The control of blood glucose levels within the normal range is recommended for the prevention of diabetic complications.10,11) Treatments with several antidiabetic agents, including insulin, and exercise training have both been shown to lower blood glucose levels in db/db mice.12-14) Fujita et al. reported that a chronic treatment with insulin reduced fasting blood glucose to normal levels in db/db mice, and this effect was more pronounced than that with metformin.12) Although findings on the lowering effects of exercise training on blood glucose levels in db/db mice have been conflicting,13,15-17) we expected positive effects, such as improvements in insulin resistance. Therefore, an insulin treatment and exercise training were herein combined to control blood glucose at normal levels and we investigated whether this prevented the exacerbation of inflammation in diabetic db/db mice.
TPA was obtained from Wako (Osaka, Japan) and TRIzol reagent from Invitrogen (Carlsbad, CA). The High Capacity cDNA Reverse Transcription Kit and pre-designed TaqMan assays (probe and primer sets) for Il1b (Assay ID Mm 01336189_m1), Il6 (Assay ID Mm00446190_m1), Tnf (Assay ID Mm00443258_m1), and VIC-labeled 18S ribosomal RNA (18S) (Assay ID Hs99999901_s1) were purchased from Applied Biosystems (Foster city, CA). RNAlater and mirVana miRNA isolation kits were obtained from Ambion (Austin, TX).
AnimalsAll experimental protocols regarding the treatment of animals were approved by the Animal Care and Use Committee of Setsunan University and conducted following the guidelines of the Japanese Pharmacological Society. Five- or eight-week-old male db/db and age-matched control (db/m) mice were purchased from Japan SLC (Shizuoka, Japan). Mice were fed standard chow and maintained under controlled environmental conditions (12-h light/dark cycle; light 07:30-19:30 hours, 23 ± 2°C, and 55 ± 10% humidity).
Measurement of Blood Glucose and HbA1c LevelsBlood glucose levels were measured in blood collected from the tail vein using a glucose test meter (Sanwa Kagaku Kenkyusho, Aichi, Japan). HbA1c concentrations in whole blood were assessed using an immunoassay system (DCA 2000; Siemens, Munich, Germany).
TPA-Induced Ear InflammationTwo micrograms of TPA dissolved in 20 µL of acetone (0.1 µg/µL) was applied to the left ear of 9-week-old mice (10 µL each for the inner and outer surfaces of the ear), while 20 µL of acetone was applied to the right ear. In the experiment on the effects of the insulin treatment combined with exercise training, TPA or acetone was applied to both ears of each mouse. Ear thickness was measured using a dial thickness gauge, as previously described.6) Animals were euthanized by cervical dislocation under isoflurane anesthesia, and both ears were harvested.
Insulin Treatment Combined with Exercise TrainingDb/db mice were divided into two groups, one of which received the insulin treatment combined with exercise training for 3 weeks daily from 6 weeks of age. Insulin (Novolin N injection, 10 U/kg) was subcutaneously administered twice daily (8:30 and 19:00). Exercise training was performed every morning for 1 h (7:30-8:30) using a mouse forced exercise wheel system (FWS-1504, MELQUEST). Exercise intensity was based on previous studies showing the beneficial effects of exercise on blood glucose levels with slight modifications.13) The initial speed was set at 4 m/min, increased daily by 1 m/min, and reached 8 m/min by the end of the first week of training. Mice were run for 1 h/day in subsequent weeks at 8 m/min. The speed setting of 8 m/min was the same as that used in treadmill exercise performed by db/db mice in other studies.18,19) The other db/db group and db/m mice received saline without exercise training.
RNA IsolationHarvested ears were immediately placed in RNAlater stabilization buffer to prevent RNA degradation. Ears were placed in RNAlater at 4°C overnight, removed from solution, and then stored at -80°C until the isolation of RNA. Frozen ears were disrupted using a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan). Total RNA (including small RNA molecules) was extracted with TRIzol reagent and the mirVana miRNA Isolation kit following the manufacturer’s protocol.
Quantification of mRNA Using Real-Time RT-PCRReverse transcription was performed using the High Capacity cDNA Reverse Transcription kit. A duplex quantitative RT-PCR assay, using TaqMan probes labeled with different dyes, was performed on a CFX96 real-time system (Bio-Rad Laboratories, Hercules, CA). mRNA levels were normalized with the expression of 18S, which was simultaneously analyzed. Relative expression levels were calculated with the 2-ΔΔCt method.
HistologyHarvested ears were immediately fixed in 4% formaldehyde. They were then embedded in paraffin, sliced into 3-µm-thick sections, mounted on slides, and stained with hematoxylin and eosin following standard protocols.
Statistical AnalysisExperimental data were expressed as means ± SEM. Statistical analyses were performed using the Student’s t-test for single comparisons and a one-way ANOVA followed by Tukey’s test for multiple comparisons. Differences were considered to be significant at P < 0.05.
At 9 weeks of age, blood glucose levels were markedly higher in db/db mice than in db/m mice (438 ± 42.5 vs. 134 ± 7.23 mg/dL). The topical application of TPA, but not acetone, induced increases in ear thickness in db/m and db/db mice (Fig. 1A). In db/m mice, ear thickness peaked at 8 h and then declined. Ear thickness did not significantly differ between db/db and db/m mice 8 h after the TPA treatment, but was significantly greater in db/db mice after 24 and 32 h (Fig. 1A). Histological observations showed that the inflammatory response, accompanied by increased exudation, was more robust in db/db mice 32 h after the TPA treatment (Fig. 1B). The TPA treatment increased the gene expression of pro-inflammatory cytokines (IL-1beta, IL-6, and TNF-alpha). Although mRNA expression levels in acetone-treated ears were significantly higher in db/db mice than in db/m mice (i.e., IL-1beta at 8 h: 4.6-fold higher, IL-6 at 8 h: 5.5-fold higher, and IL-6 at 24 h: 3.0-fold higher), these differences were smaller than the alterations induced by TPA treatment (Fig. 1C). Therefore, low-grade systemic inflammation in db/db mice did not affect TPA-induced inflammation. In db/m mice, the expression of the pro-inflammatory cytokines tested peaked 8 h after the TPA treatment and then declined after 24 and 32 h (Fig. 1C). In db/db mice, the gene expression levels of IL-1beta and IL-6 did not significantly differ from those in db/m mice 8 h after the TPA treatment, but were significantly higher after 24 and 32 h (Fig. 1C). In addition, the gene expression levels of TNF-alpha were significantly higher in db/db mice 8, 24, and 32 h after the TPA treatment (Fig. 1C).
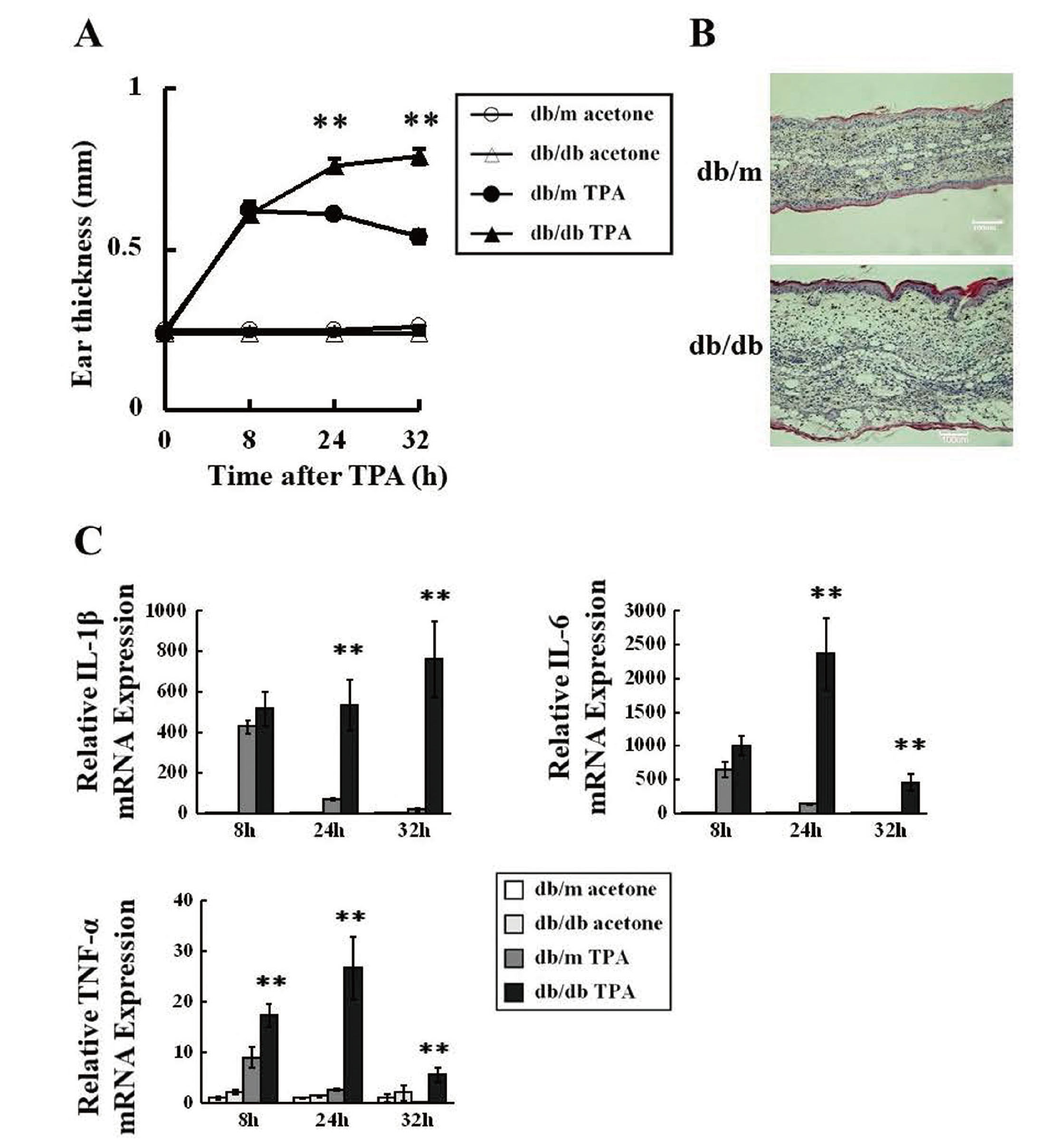
TPA-Induced Ear Inflammation Was Exacerbated in Diabetic db/db Mice
TPA (2.0 µg) or acetone (vehicle) was applied to the ears of control db/m and diabetic db/db mice. A, Ear thickness 8, 24, and 32 h after the TPA treatment. B, Ear sections from db/m and db/db mice 32 h after the TPA treatment. C, Altered expression profiles of mRNAs during TPA-induced inflammation. Quantitative real-time RT-PCR assessed IL-1beta, IL-6, and TNF-alpha mRNA expression with total cDNA derived from the acetone- or TPA-treated ears of db/m and db/db mice. Values are the mean ± SEM; n = 4. **P < 0.01, vs. db/m mice (TPA-treated).
The insulin treatment combined with exercise training did not significantly change the body weight of db/db mice (Fig. 2A). However, it suppressed blood glucose levels in 8- and 9-week-old db/db mice. In addition, HbA1c levels were significantly suppressed in 9-week-old db/db mice by the combination of the insulin treatment and exercise training (Fig. 2C). Blood glucose and HbA1c levels were similar in 9-week-old treated db/db mice and db/m mice (Fig. 2B and C).
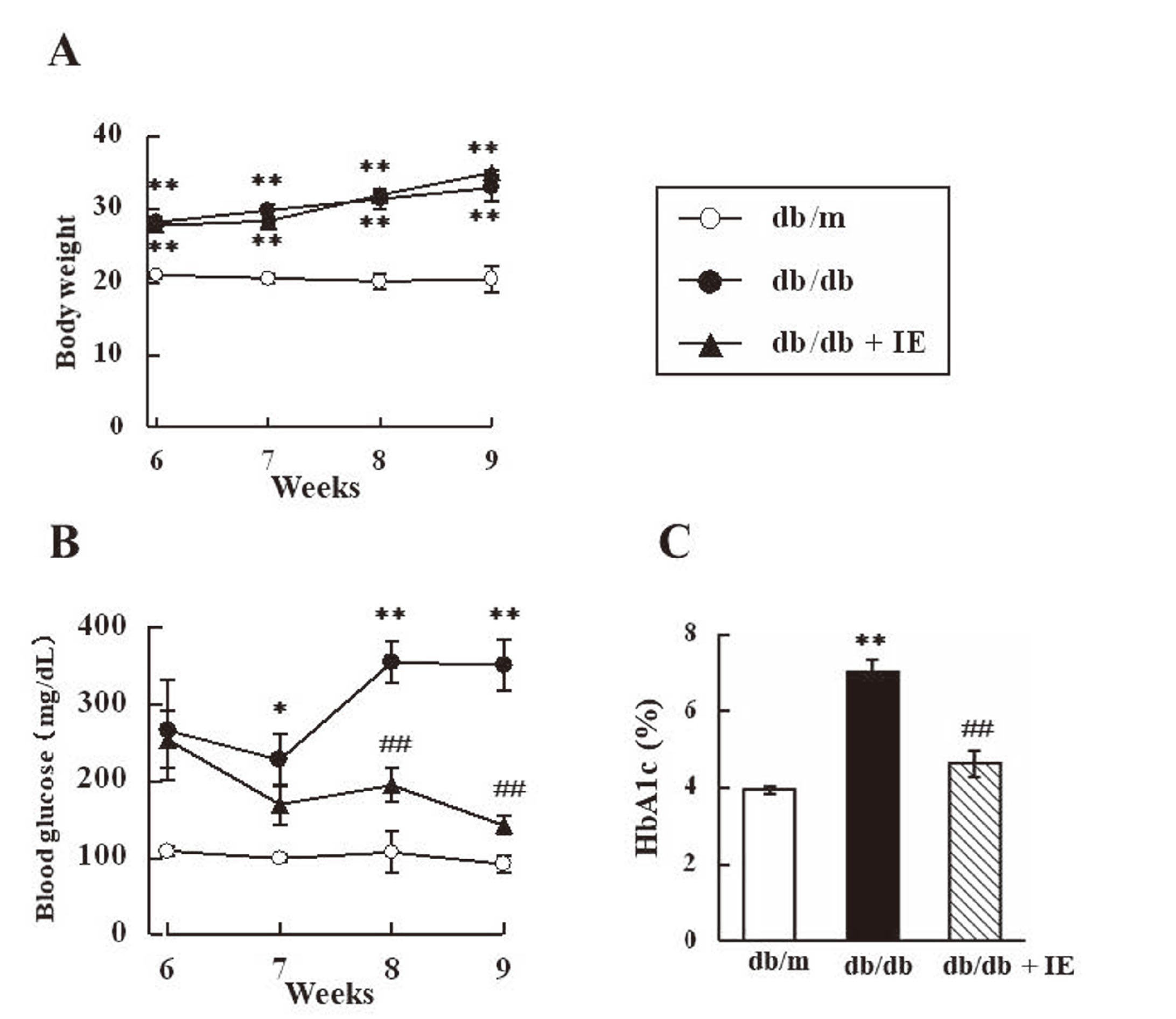
Effects of the Insulin Treatment Combined with Exercise Training on Body Weights, Blood Glucose Levels, and HbA1c Levels in db/db Mice.
db/db mice were divided into two groups, one of which received the insulin treatment combined with exercise training for 3 weeks daily from 6 weeks of age (db/db+IE). Insulin (Novolin N injection, 10 U/kg) was subcutaneously administered twice daily, and exercise training was performed every morning for 1 hour. The other db/db group and db/m mice received saline without exercise training. A and B, Weekly changes in body weights and blood glucose levels in db/m, db/db, and db/db+IE mice. C, HbA1c levels in 9-week-old db/m, db/db, and db/db+IE mice. Values are the mean ± SEM; n = 4. **P < 0.01, vs. db/m mice. ## P < 0.01, vs. db/db mice.
The insulin treatment combined with exercise training did not affect ear thickness 32 h after the TPA treatment in db/db mice (Fig. 3A). Consistent with its effects on ear thickness, the insulin treatment combined with exercise training did not suppress the up-regulated expression of IL-1beta, IL-6, and TNF-alpha 32 h after the TPA treatment in db/db mice (Fig. 3B).

The Insulin Treatment Combined with Exercise Training Did not Prevent the Exacerbation of TPA-Induced Inflammation in db/db Mice
db/db mice were divided into two groups, one of which was given the insulin treatment combined with exercise training for 3 weeks daily from 6 weeks of age (db/db+IE). Insulin (Novolin N injection, 10 U/kg) was subcutaneously administered twice daily, and exercise training was performed every morning for 1 hour. The other db/db group and db/m mice received saline without exercise training. TPA (2.0 µg) was applied to the ears of 9-week-old db/m, db/db, and db/db+IE mice. A, Ear thickness 32 h after the TPA treatment in db/m, db/db, and db/db+IE mice. B, The mRNA expression of IL-1beta, IL-6, and TNF-alpha 32 h after the TPA treatment in db/m, db/db, and db/db+IE mice. The other 9 weeks old, db/m and db/db mice, were prepared for acetone application. Values are the mean ± SEM; n = 4. *P < 0.05, **P < 0.01, vs. db/m mice (TPA-treated).
The degree of ear swelling and histological observations indicated that the severity of TPA-induced inflammation was significantly higher in db/db mice than in db/m mice (Fig. 1A and B). Furthermore, the mRNA expression levels of inflammatory cytokines in TPA-treated ears were higher in db/db mice (Fig. 1C). These results are consistent with previous findings on STZ-induced diabetic mice; however, the extent of the exacerbation was higher in db/db mice than in STZ-induced diabetic mice. Ear inflammation induced by TPA is one of the well-known models of acute inflammation,20) in which the entire process from pre-onset to the resolution of inflammation may be easily observed based on ear thickness. The present results showed that TPA-induced inflammation was exacerbated in diabetic db/db mice. Similar findings may be obtained in other models of acute inflammation. Previous studies reported that LPS or ozone-induced inflammatory responses were exacerbated in db/db mice.21, 22)
Although wound healing is known to be delayed in STZ and db/db mice, the underlying mechanisms remain unclear.7) Tissue repair after skin injury involves the clearance of apoptotic cells at the wound site by phagocytes via efferocytosis.23,24) Defects in efferocytosis are associated with non-resolving inflammation, leading to chronic inflammatory conditions.23,24) Maschalidi et al. recently reported that the membrane transporter SLC7A11 acted as a molecular brake on efferocytosis and also that the inhibition of SLC7A11 functions may accelerate wound healing in db/db mice.23) Although many factors affect the wound healing process, abnormally prolonged or augmented inflammation in the wound site is a common cause of poor wound healing.23-26) Although there is no direct relationship between TPA-induced inflammation and wound healing, we used TPA-induced inflammation as a simple experimental model of the inflammatory phase of the wound healing process.6) TPA-induced inflammation may provide insights into the effects of DM on the inflammatory phase of wound healing because this model rules out its impact on other steps in the wound healing process. No significant differences were observed in ear thickness or the mRNA levels of IL-1beta and IL-6 alpha between db/db and db/m mice 8 h after the TPA treatment (Fig. 1A and C). These results suggest that the exacerbation of TPA-induced inflammation in db/db mice was due to impairments in the resolution, but not initiation, phase of inflammation. In addition, accumulating evidence suggests that impaired efferocytosis is responsible for delayed wound healing in db/db mice.23,24,27,28) Therefore, the exacerbation of TPA-induced inflammation may also be caused by impaired efferocytosis. However, it remains unclear whether the better control of blood glucose levels promotes impaired efferocytosis and prevents the exacerbation of inflammation in DM.
In the present study, we examined the effects of glucose-lowering interventions to reveal the involvement of hyperglycemia in exacerbated inflammation in db/db mice. The insulin treatment combined with exercise training significantly suppressed blood glucose levels, but did not prevent the exacerbation of TPA-induced inflammation in db/db mice (Fig. 2 and 3). Since the administration of insulin to normal animals may have reduced blood glucose to below normal levels, a db/m+IE group was not established in the analysis shown in Fig. 2. Pietramaggiori et al. previously reported that the parabiotic joining of wild-type and db/db mice improved wound healing in db/db partners without affecting glycemic control.29) In addition, no correlation was observed between the wound closure rate and fasting blood glucose levels in db/db mice.30,31) These findings suggest that wound healing in db/db mice is independent of blood glucose levels, which is consistent with the present results. Although the American Podiatric Medical Association and Society for Vascular Medicine now recommend adequate glycemic control to reduce the incidence of diabetic foot ulcers, there is currently no compelling evidence to support tight glycemic control for the promotion of wound healing.4,5)
Since protective effects were not observed with combination therapy, we did not examine the impact of insulin treatment or exercise training alone in the present study. Accumulating evidence suggests that systemic insulin treatment has no positive effects on diabetic wound healing.32-35) In contrast, the topical application of insulin promoted wound healing in diabetic humans and animals.36,37) In addition, despite the millions of patients taking insulin, large-scale clinical trials have not yet been performed on the effects of systemic insulin treatment on diabetic wound healing.38) There have also been conflicting findings on the impact of exercise training on body weight and blood glucose levels in db/db mice.13,15-17,39-41) The reason for this discrepancy remains unclear, but may be due to differences in exercise duration and intensity. Keylock et al. reported that low-, but not high-intensity exercise accelerated wound healing in db/db mice.42) Although the method of exercise loading selected in the present study was based on previous studies, we cannot rule out the possibility that the exercise load was insufficient; db/db mice passively performed the wheel running exercise, they did not appear to actively walk. Veerank et al. reported that the majority of db/db mice were unmotivated and needed assistance in the form of a gentle push on their backs using a brush throughout the treadmill exercise period.43) Therefore, forced swimming may be a better option for future exercise loading in db/db mice because it reduces the load caused by body weight. Exercise training has been associated with increased insulin sensitivity and better glucose tolerance.44) However, since insulin sensitivity was not measured in the present study, it remains unknown whether insulin resistance was attenuated.
In conclusion, TPA-induced ear inflammation was exacerbated in db/db mice. TPA-induced inflammation may be a suitable model for investigating about prolonged or augmented inflammation in diabetic mice. In addition, the insulin treatment combined with exercise training did not prevent the exacerbation of TPA-induced inflammation in db/db mice.
Conflict of interestThe authors declare no conflict of interest.