2023 Volume 6 Issue 3 Pages 122-125
2023 Volume 6 Issue 3 Pages 122-125
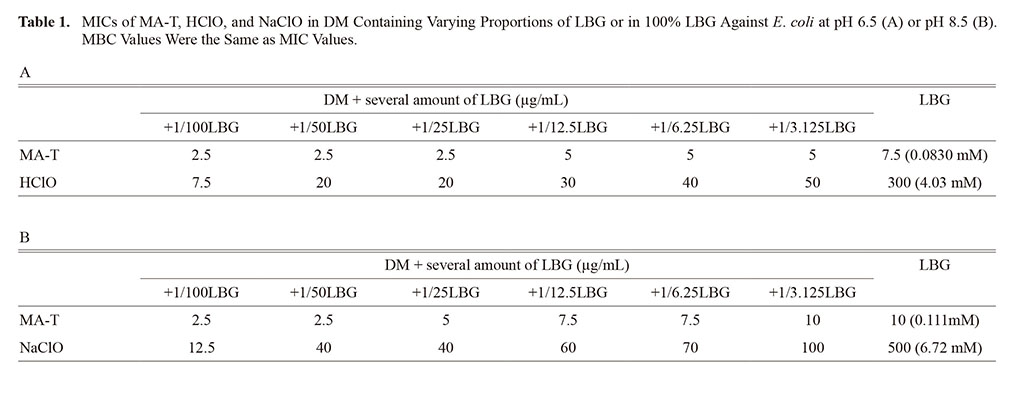
Effectiveness of disinfectant MA-T against Escherichia coli in various growth media was demonstrated by minimal inhibitory concentration (MIC). MIC of MA-T in (DM+1/100 volume of LBG [Luria-Bertani medium with 0.4% glucose]) at pH 6.5 and pH 8.5 was 2.5 μg/mL, and that in 100%LBG at pH 6.5 and pH 8.5 was 7.5 μg/mL and 10 μg/mL, respectively. MA-T is not markedly affected by organic materials or pH. MIC of HClO in DM+1/100LBG at pH 6.5 and pH 8.5 was 7.5 μg/mL and 12.5 μg/mL, respectively, but MIC in rich medium (100%LBG) at pH 6.5 and pH 8.5 was 300 μg/mL and 500 μg/mL, respectively, indicating that HClO does not affect bacteria because of preferential reaction with biological materials contained in LBG, with minimal difference at varying pH. Growth of E. coli as monitored in aerobic shaking culture in LBG was dramatically reduced by adding 25 μg/mL MA-T, but growth was not affected by 100 μg/mL HClO (pH 6.5) or NaClO (pH 8.5). The CFU/mL of E. coli in aerobic standing culture in LBG declined linearly with incubation time on a logarithm chart after addition of 25 μg/mL MA-T. Viability was not affected by the addition of 100 μg/mL HClO (pH 6.5) or NaClO (pH 8.5). In conclusion, bactericidal effect of MA-T against E. coli is minimally affected by biological substances at different pH values (pH 6.5 or 8.5), but bactericidal effect of both HClO (pH 6.5) and NaClO (pH 8.5) is completely abolished by biological substances.
The public health importance of suppressing the spread of infectious diseases has continued to increase over the last several decades. Numerous emerging and re-emerging infectious diseases exist, including drug-resistant tuberculosis, methicillin-resistant Staphylococcus aureus–derived diseases, cholera caused by Vibrio cholerae, hemorrhagic colitis caused by Escherichia coli (EHEC), and coronavirus disease–2019 (COVID-19).1,2) There is a clear need for effective disinfectants capable of inactivating microorganisms without adversely affecting the human body. Chlorine dioxide and chlorine, common bleaching and disinfecting chemicals used in the food industry, are potent chlorinating agents. Chlorine dioxide, a reagent with strong oxidizing ability, is one of the most efficient disinfectants available.3) The application of chlorine dioxide as a pre-oxidant, instead of chlorine, carries the benefit of minimizing post-chlorination trihalomethane production.4,5) The ClO2 radical is a powerful one-electron oxidant, exhibiting a redox potential of 936 mV,6) and it is known for its ability to oxidize both inorganic and organic species.6,7) ClO2 triggers the denaturation of enzymes and other proteins,8) which disrupts metabolic pathways via protein and metabolite modification to kill a variety of microorganisms, including bacteria, fungi, and viruses.
The matching transformation system (MA-T) is an on-demand aqueous chlorine dioxide solution in which the generation of aqueous radicals is controlled by an organic catalyst.9) MA-T exhibits outstanding safety and varied antimicrobial effects, as we previously reported.10-13) MA-T provides sufficient antimicrobial effects at 100 μg/mL even in medium rich in organic matter, and it was shown to be safe at the same or even higher concentrations, regardless of exposure route.10,11) MA-T is not flammable, volatile, or corrosive, so it is expected to play a beneficial role in situations in which conventional disinfectants cannot be used.11)
Another agent with a mechanism of action involving radical formation is hypochlorous acid (HClO at acidic pH, the pKa of this acid is pH 7.5) or hypochlorite ion (ClO− at basic pH, usually used as NaClO), which is widely known.14) This agent is utilized in many fields as an oxidizer, bleaching agent, and disinfectant. However, the strongly irritating nature of hypochlorite is a disadvantage, which limits its use in liquid form and necessitates careful handling due to its toxicity.15) HClO is reportedly a more effective disinfectant than NaClO against many bacteria.16,17) However, HClO and NaClO are readily inactivated in the presence of biological substances or organic matter at neutral pH.18)
In this study, we demonstrated that the bactericidal effect of MA-T against Escherichia coli is minimally affected by biological substances or organic matter contained in rich medium at different pH values (pH 6.5 and 8.5). However, the antibacterial effect of both HClO (pH 6.5) and NaClO (pH 8.5) was completely abolished by biological substances.
Escherichia coli MV1184 derived from strain K12 (in our laboratory collection) was aerobically standing cultured in the wells of a 96-well microplate (200 μL per well) at 37°C. Davis minimal medium19) (DM) for culturing E. coli is free of biological substances and organic matter and consists of 0.3% (22.05 mM) KH2PO4, 0.7% (40 mM) K2HPO4, 0.05% trisodium citrate–3H2O, 0.1% (NH4)SO4, 0.01% MgSO4, and 0.2% glucose. The concentrations of glucose, KH2PO4, and K2HPO4 were changed to 0.4%, 34 mM, and 16 mM, respectively, for the preparation of DM (pH 6.5). The concentration of glucose was changed to 0.4%, KH2PO4 was removed, 7.5 mM NaOH was added, and the amount of K2HPO4 was modified to 50 mM for DM (pH 8.5). In practice, E. coli cannot grow in DM, as the water and chemical reagents used for culture medium are generally highly pure and contain very limited amounts of essential trace metals such as iron and copper ions. In addition, as strain MV1184 requires thiamine because of a mutation in the thy gene, a 1/100 volume of LBG (Luria-Bertani medium with 0.4% glucose) was added to DM to prepare an available minimal medium for our experiments. LGB consists of 1% polypeptone, 0.5% yeast extract, 0.5% NaCl, and 0.5% glucose, and 5 mM KH2PO4 was added to LBG for pH 6.5, and 10 mM K2HPO4 and 5 mM NaOH was added for LBG at pH 8.5. Media richer than DM + 1/100 LBG were prepared by increasing the amount of LBG, such as DM + 1/50 LBG, DM + 1/25 LBG, DM + 1/12.5 LBG, DM + 1/6.25 LBG, and DM + 1/3.125 LBG. The richest medium was 100% LBG. MICs were determined using standing culture in microplates at 37°C.
Bacterial Growth Curves After Addition of MA-T, HClO, or NaClO at pH 6.5 and pH 8.5Escherichia coli was cultured at 37°C with vigorous shaking in LBG medium at pH 6.5 and pH 8.5. Bacterial growth was monitored by measuring the absorbance at 650 nm at regular intervals using a spectrophotometer. MA-T or NaClO was added in the mid-logarithmic phase (indicated by arrows in Fig. 1).
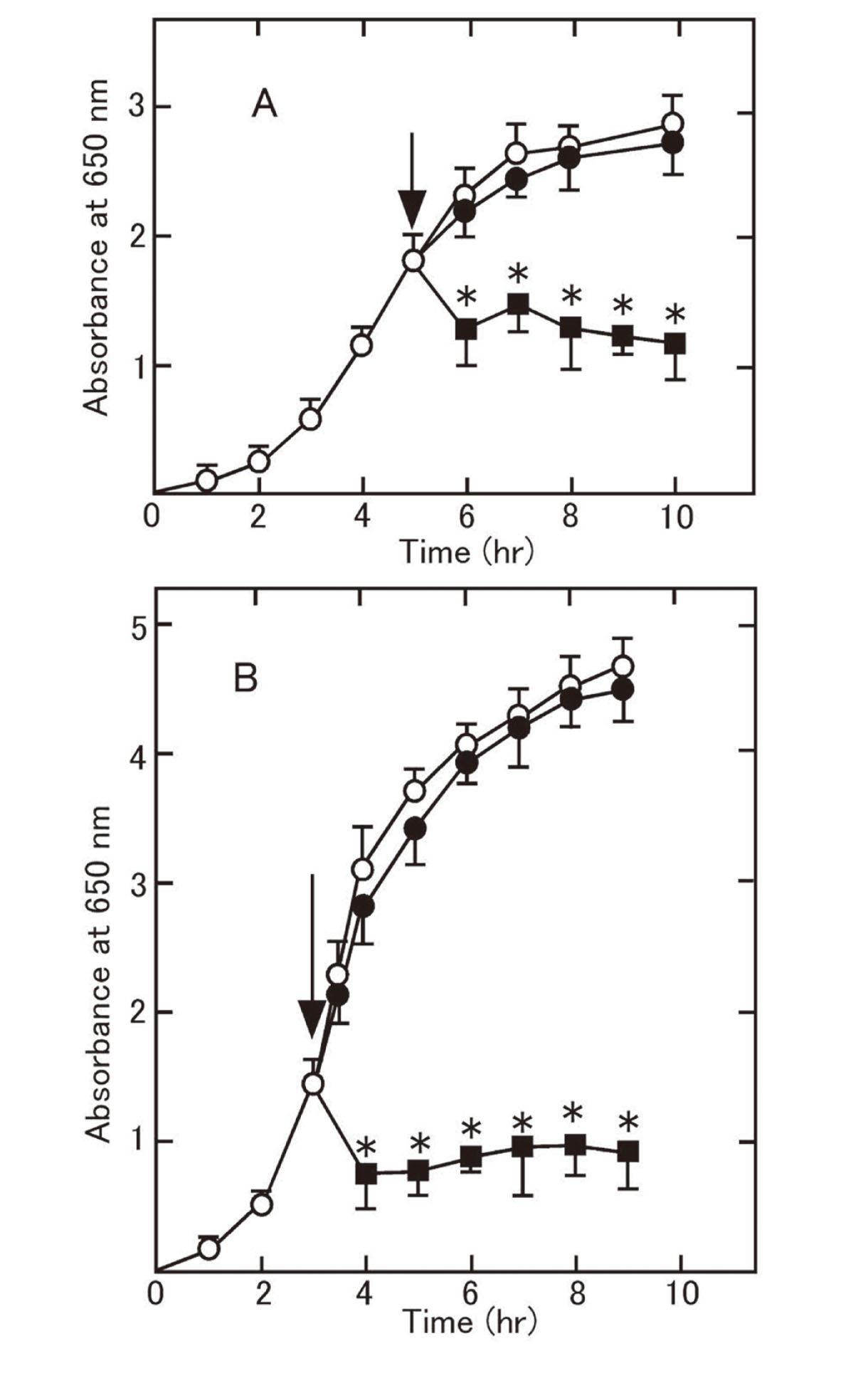
Absorbance at 650 nm of E. coli Cultured in LBG Medium
Disinfectant (A: at pH 6.5; B: at pH 8.5) was added at the time indicated by the arrow. Asterisks (*) indicate a significant decrease in absorbance when compared to no addition of disinfectant at corresponding growth time. Each data point is the mean ± SD (n=6). Closed circles (absorbance after addition of 100 μg/mL HClO [A: pH 6.5] or 100 μg/mL NaClO [B: pH 8.5]), closed squares (absorbance after addition of 25 μg/mL MA-T [A: pH 6.5] or 25 μg/mL MA-T [B: pH 8.5]), open circles (absorbance after no addition at pH 6.5 [A] or pH 8.5 [B] as controls).
As indicated above, E. coli was cultured at 37°C with vigorous shaking in LBG medium. An aliquot of bacterial culture medium was diluted with LBG medium to adjust the concentration of bacteria, disinfectant was added at time 0, and the cells were standing cultured at 20°C. The number of CFU (colony forming units)/mL was measured after disinfectant treatment by serially diluting the treated cultures with phosphate-buffered saline (PBS), spreading aliquots on brain heart infusion agar (Beckton Dickinson and Company, MD, USA) plates, incubating overnight at 37°C, and counting the number of colonies formed per plate.
Statistical AnalysisAll assays were performed as two independent experiments (biological replicates), each consisting of triplicate reactions (technical replicates; total n = 6). Unless otherwise indicated, values are presented as the mean ± standard deviation (SD). The significance of differences was determined using the two-tailed, non-paired Student’s t test. Differences were considered significant at p<0.05 (shown by an asterisk). All data were analyzed using StatMate (Atms Co., Ltd., Tokyo, Japan).
The molecular concentration ratio of acid and corresponding base of hypochlorous acid is given by the Henderson-Hasselbalch equation (log [HClO]/[ClO−] = pKa – pH). As log [HClO]/[ClO−] = 1 at pH 6.5 and −1 at pH 8.5, the relative number of HClO and ClO− molecules is 10 times and 1/10, respectively (calculated by pKa = 7.5).20) In general, MIC and minimal bactericidal concentration (MBC) values are determined after 1 day of culture at 37°C for E. coli. However, it is very difficult to determine MIC and MBC values in a poor medium such as DM, as the growth of E. coli in poor medium is very slow, requiring 2 or 3 days for notable turbidity to appear in the culture medium. MIC values in the presence of disinfectant were consequently determined after 2 or 3 days of culture from the start of the experiment, and MBC values were determined by spreading 1-10 μL of the culture on BHI ager plates, with colony formation observed after an additional 1 day of culture. Agreement between MIC and MBC values was observed in our experiments, as the culture time was long (2 or 3 days).
The effectiveness of MA-T against E. coli in various growth media can be seen from the MIC values shown in Table 1. The MIC of MA-T in DM + 1/100 LBG at pH 6.5 was 2.5 μg/mL, and this value was slightly increased by further increasing the ratio of LBG to DM. The MIC of 100% LBG was 7.5 μg/mL at pH 6.5. In contrast, the MIC of HClO in poor medium (DM + 1/100 LBG) at pH 6.5 was 7.5 μg/mL. The MIC increased to 300 μM in rich medium (100% LBG) at pH 6.5, indicating that HClO cannot interact with the bacteria due to preferential reaction with biological materials contained in LBG. The MIC of MA-T in DM + 1/100 LBG at pH 8.5 was 2.5 μg/mL and increased with further addition of LBG to DM. The MIC of 100% LBG was 10 μg/mL at pH 8.5. The MIC of NaClO in DM + 1/100 LBG at pH 8.5 was 12.5 μM, but the MIC increased to 500 μg/mL in 100% LBG at pH 8.5. The difference in MIC between HClO (pH 6.5) and NaClO (pH 8.5) was approximately 50%, indicating that hypochlorite ion is less effective agents than HClO as reported in the literature.16,17) On the other hand, MA-T was not affected by either organic materials in LBG or pH.
The effect of the disinfectants on the growth of cultured E. coli was examined. The absorbance of the bacterial culture medium, LBG, was measured at 650 nm following the addition of disinfectant in the mid-logarithmic phase, as shown in Fig. 1. Growth was inhibited by 25 μg/mL MA-T at pH 6.5 and pH 8.5 but not significantly affected by 100 μg/mL HClO (pH 6.5) or 100 μg/mL NaClO (pH 8.5). The presence of biological materials in LBG medium abolished the inhibition of bacterial growth.
The viability of E. coli cells in aerobic standing culture at 20°C in LBG medium was analyzed by determining the colony forming capacity after the addition of MA-T, HClO, or NaClO. As indicated in Fig. 2, the number of CFU/mL in the presence of 25 μg/mL MA-T at pH 6.5 and pH 8.5 decreased linearly with incubation time on a semi-logarithmic plot. The D value (decimal reduction value, or time required for a 1-log reduction) was 4.32 min (pH 6.5) and 5.07 min (pH 8.5) as determined by linear regression analysis, indicating that the log reduction induced by MA-T is not significantly affected by pH. However, the viability of the bacteria was not reduced by the addition of HClO (pH 6.5) or NaClO (pH 8.5), regardless of bacterial density (log [CFU/mL] = 8.4 or 7.4) at 0 min. These results revealed that the bactericidal effect of HClO and NaClO is completely abolished by biological substances contained in LBG medium, such as polypeptone and/or yeast extract.

Inactivation of E. coli in LBG Medium in the Presence of Disinfectant (100 μg/mL HClO [pH 6.5] or NaClO [pH 8.5] or 25 μg/mL MA-T) as a Function of Time
Open triangle (log [CFU/mL]=8.4) and closed triangle (log [CFU/mL]=7.4): 100 μg/mL HClO (pH 6.5), open square (log [CFU/mL]=8.4) and closed square (log [CFU/mL]=7.4): 100 μg/mL NaClO (pH 8.5), open circles: 25 μg/mL MA-T (pH 6.5), closed circles: 25 μg/mL MA-T (pH 8.5). Asterisks (*) indicate statistically significant difference compared with time 0 control log CFU/mL (p<0.05). Each data point represents the mean ± SD (n=6). The D values (decimal reduction value, or time required for a 1-log reduction) were 4.32 min (MA-T, pH 6.5) and 5.07 min (MA-T, pH 8.5).
This study was supported by grant no. JPMJOP1861 from the Program on Open Innovation Platform with Enterprises, Research Institute and Academia (OPERA), Japan Science and Technology Agency (JST). The URL of JST OPERA is https://www.jst.go.jp/opera/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of InterestTakekatsu Shibata is a director of Acenet Inc., which manufactures the MA-T. The other authors declare no conflict of interest.