2023 Volume 6 Issue 3 Pages 76-80
2023 Volume 6 Issue 3 Pages 76-80
Semi-volatile organic compounds (SVOCs), which can cause indoor air pollution, include plasticizers, insecticides, and flame retardants. In Japan, the Ministry of Health, Labour and Welfare has set guidelines for indoor air concentrations of di-n-butyl phthalate and di-2-ethylhexyl phthalate in plasticizers and fenobucarb, diazinon, and chlorpyrifos in insecticides. However, this analytical method has only been tentatively proposed for more than 20 years. In this study, we attempted to construct an analytical method for insecticides for which guideline values have been established based on recently standardized sampling and extraction methods for phthalates in indoor air. The results of the recovery tests for the insecticides were excellent, with recovery rates and relative standard deviations in the ranges 88%–104% and 1.4%–7.5%, respectively. Furthermore, the limits of detection and quantification were less than 1/50 of the current guideline values. Additionally, inter-laboratory validation was conducted at five research institutions. By excluding outliers with the Grubbs test, the accuracies were in the ranges of 81.9%–126.3%, 76.8%–121.7%, and 76.7%–112.8% for chlorpyrifos, diazinon, and fenobucarb, respectively. The target ranges for repeatability (RSDr) and reproducibility (RSDR) were 30% and 35%, respectively, and the validation results met these criteria. Based on these results, we propose the developed method as the standard test method for insecticide-originated pollutants in indoor air in Japan.
Semi-volatile organic compounds (SVOCs) have a boiling point of 240–400°C and are characterized by a lower vapor pressure than volatile organic compounds.1) The sources of SVOCs are plasticizers, flame retardants, and insecticides in furniture, building materials, and household products, and are ubiquitous in any indoor environment.2) Therefore, SVOCs contaminate indoor air through gradual and continuous emissions.3)
Moreover, some SVOCs can cause illnesses, such as sick-building syndrome and asthma.4–5) In Japan, the name “sick-house syndrome” has become popular because of the high incidence of health hazards, particularly in residential areas.6) As a countermeasure for indoor air pollution, guideline values for indoor air concentrations were set for five SVOCs from 2000 to 2002. The five SVOCs included two phthalates, di-n-butyl phthalate (DnBP) and di-2-ethylhexyl phthalate (DEHP), and three insecticides: fenobucarb, diazinon, and chlorpyrifos. Fenobucarb is a carbamate insecticide, whereas diazinon and chlorpyrifos are classified as organophosphorus insecticides. These substances cause cholinesterase inhibition and affect the brain and nervous system.7–9) In Japan, chlorpyrifos was widely employed as a termite control building material until it was banned in 2003 because of its high neurotoxicity.10)
Over 20 years have passed since guideline values for indoor concentrations have been established in Japan. The Committee on Indoor Air Pollution (CIAP) established the current, official sampling, and analytical method for chemical substances in indoor air in 2001.11) The Ministry of Health, Labour and Welfare (MHLW) of Japan continually reviews the values and considers new chemical substances for inclusion. For instance, in 2019, the MHLW revised the guideline values for DnBP and DEHP to lower values.12) However, a review of the analytical method of SVOCs in indoor air remains largely unestablished. The current official method is only a tentative proposal because it has yet to be validated, although sampling and analytical methods are provided.
There are two common methods for measuring the concentration of SVOCs in indoor air: solvent extraction (SE) and thermal desorption (TD).13,14) The SE method can be conducted using a variety of adsorbents and requires no special equipment other than gas chromatograph. However, this method has poor sensitivity and efficiency. In contrast, in the TD method, compounds collected in a dedicated adsorbent tube, such as “Tenax®,” are introduced directly into the gas chromatograph. This method requires no extraction and is highly sensitive; however, the equipment is expensive and reanalysis is difficult. Thus, among both methods, SE is considered a versatile approach and is currently the most utilized for indoor air analysis.
Based on the above, we decided to use the versatile SE method and review the official methods for insecticides according to technological advances in sampling and analytical instruments. Kagawa et al. recently devised a “standard test method for phthalates” (SMP) in indoor air based on SE.14) In the SMP, an air-sampling cartridge consisting of a quartz filter and a styrene–divinylbenzene copolymer (SDB) cartridge was utilized. Furthermore, this method is simple, and extraction can be achieved using acetone alone. Thus, the sampling and extraction of phthalates using an SDB cartridge may promote insecticide analysis, for which guidelines for indoor air concentrations have been established. In this study, we developed an analytical method for insecticide detection based on the SMP.
Chlorpyrifos, diazinon, and fenobucarb were purchased from AccuStandard Co. (CT, USA). Chlorpyrifos-d10 (Kanto Chemical Co., Tokyo, Japan) was used as an internal standard. Acetone for the pesticide residue and polychlorinated biphenyl tests were purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan). AERO LE Cartridge SDB400HF was purchased from GL Science Inc. (Tokyo, Japan), and Minipump MP-∑300NII (Sibata Scientific Technology LTD., Tokyo, Japan) was used as the air-sampling pump.
Analytical MethodsDetails of the analyses are presented in Table 1. All gas chromatograph–mass spectrometer (GC–MS) quantitative analyses were performed in selected-ion monitoring mode, and the quantification and confirmation ions are shown in Table 2. The calibration curve ranges were set to 1–100 ng/mL for chlorpyrifos and 4–400 ng/mL for diazinon and fenobucarb. The concentration of the internal standard was 100 ng/mL for chlorpyrifos-d10.
 Air Sampling and Extraction
Air Sampling and Extraction
The cartridge was attached to a sampling pump and indoor air was aspirated at 1 L/min for 24 h (1.44 m3). The quartz filter and adsorbent were transferred to a 10 mL centrifuge tube and sonicated in 5 mL acetone for 20 min. Subsequently, the sample was centrifugaed (1740 × g, 10 min), and 1 mL of the supernatant was collected in a GC vial, to which 100 µL of the internal standard was added (Fig. 1). To address the poor sensitivity of GC–MS, 2 mL of supernatant remaining after centrifugation was concentrated to 0.5 mL with N2 gas. The supernatant was concentrated using a graduated concentration tube. After concentration, 50 µL of the internal standard was added, and GC–MS analysis was performed (Fig. 1).
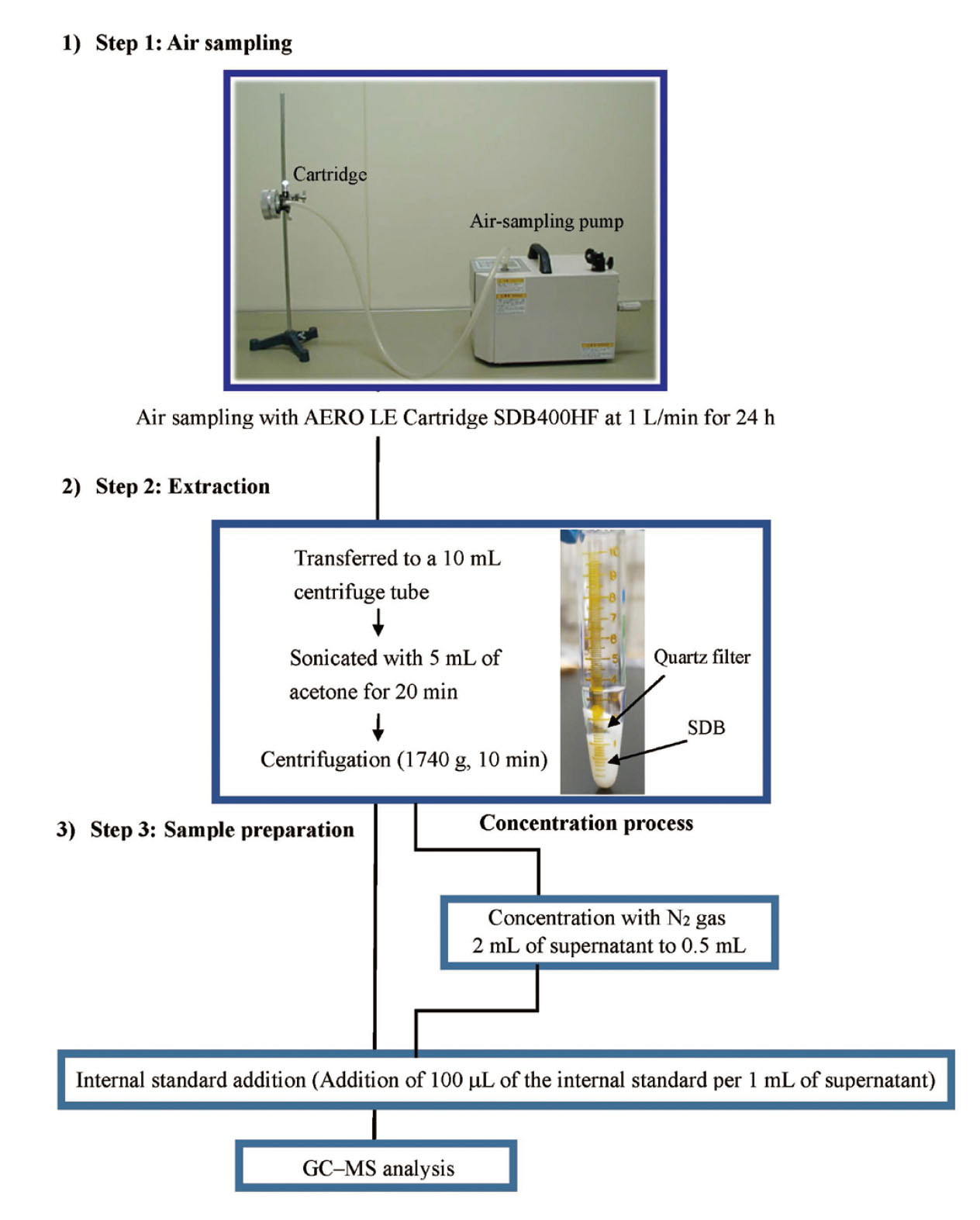
Air Sampling and Extraction Method for Insecticides in Indoor Air Using the “Standard Test Method for Phthalates (SMP).”
The standard mixture of chlorpyrifos (0.1 µg/mL), diazinon (0.35 µg/mL), and fenobucarb (0.5 µg/mL) were prepared and loaded into the cartridge (100 µL). Chlorpyrifos (10 ng), diazinon (35 ng), and fenobucarb (50 ng) were applied to SDB cartridges. In this study, all the standards were dissolved in acetone. Spiked cartridges were aspirated at 1 L/min for 24 h. At that time, air aspiration (n = 6) and without air aspiration (n = 3) were performed and analyzed by GC–MS. The LOD and LOQ were estimated to be three-fold and ten-fold the standard deviation of the concentration obtained in the recovery test (Table 3). For the blank test, the cartridge without addition was aspirated at 1 L/min for 24 h.
 Interlaboratory Validation of the Analytical Methods for the Insecticides
Interlaboratory Validation of the Analytical Methods for the Insecticides
Interlaboratory validation of the analytical methods for insecticides was conducted at five Institutes: The National Institute of Health Sciences (Kanagawa, Japan), Hokkaido Institute of Public Health (Hokkaido, Japan), Tokyo Metropolitan Institute of Public Health (Tokyo, Japan), Yokohama City Institute of Public Health (Kanagawa, Japan), and Kanagawa Prefectural Institute of Public Health (Kanagawa, Japan). These Institutes are listed in no specific order. Mixture standards, internal standards, and cartridges were distributed to each institute and their concentrations were masked. Precision was expressed as repeatability (RSDr) and reproducibility (RSDR). A series of tests was conducted, and the recovery rates were determined.
Briefly, 100 µL of the standard mixture was spiked into a cartridge and allowed to dry at room temperature for at least 30 min. Seven spiked samples were prepared and five samples were passed through indoor air at 1 L/min for 24 h. The remaining two samples were left to stand for 24 h. Blanks were prepared as cartridges with and without air aspiration. The decision on the concentration process was left to the judgment of each institution, considering the condition of the instrument. The GC–MS analysis conditions for each laboratory are listed in Table 4. Homogeneity of the test samples was verified before distribution (data not shown).
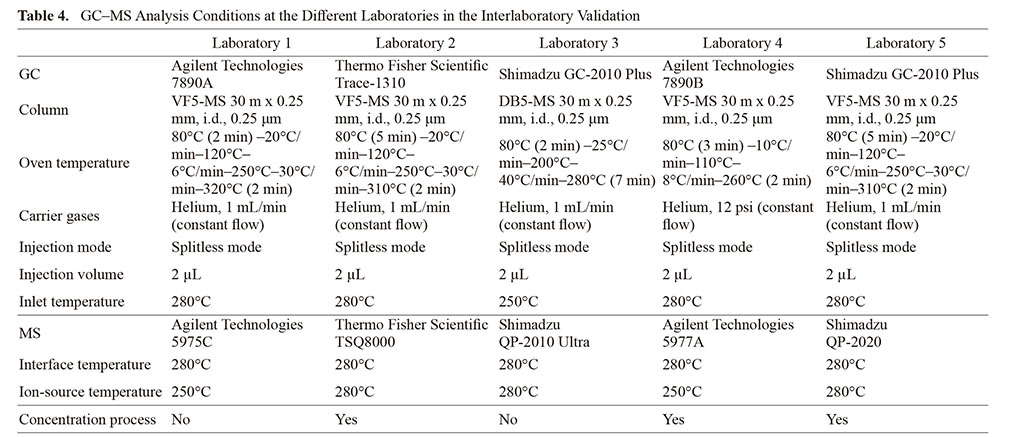 Statistical Analysis
Statistical Analysis
Twenty-five data items (five portions evaluated at five laboratories) were used in the calculations (α = 5.0%). The outliers were identified by performing a Grubbs test from the concentration data (n = 25) of all laboratories (Table S2). The use of the statistical test to identify outliers was following references 17 and 18.
Our group examined the SMP in indoor air in Japan.14,15) Adapting the SMP to the extraction method for insecticides would simplify and improve its analysis efficiency. However, the guideline values for the three insecticides range from 0.1 to 33 µg/m3, which are lower than the values for phthalates (17–100 µg/m3), raising concerns about insufficient sensitivity due to the adoption of the SMP. We examined the extraction-based analysis method for insecticides using the SMP (Fig. 1).
The guideline values for insecticides were 0.1, 0.29, and 33 µg/m3 for chlorpyrifos (for children), diazinon, and fenobucarb, respectively. The concentration (ng/mL) corresponding to 1/10 of the guideline value was calculated. The concentrations below 1/10 of the guideline value were 1.0, 4.0, and 4.0 ng/mL for chlorpyrifos, diazinon, and fenobucarb, respectively, and standard solutions of each concentration were analyzed by GC–MS. For all cases, good peaks were observed, with signal-to-noise (S/N) ratios exceeding 100 (Table S1). The calibration curves were set in the range of 1–50 ng/mL for chlorpyrifos, and 4–200 ng/mL for diazinon and fenobucarb. Good linearity was obtained, with a coefficient of determination (R2) exceeding 0.99 (Table S1).
Next, we evaluated the performance of the analytical method by conducting a recovery test. Notably, air-sampling cartridges collect many compounds, which may affect quantification accuracy. Furthermore, matrix effects, such as ionization-enhancing or -suppressing effects, are frequently observed, particularly during MS quantitation. Thus, we evaluated the recovery rate with and without aspiration into the cartridge to accurately quantify the trace amounts of insecticides in the air. Without air aspiration into the cartridge, the recovery rate was in the range of 78%–108%, and the relative standard deviation (RSD) was in the range of 3.5%–5.8% (mean of n = 3). With air aspiration, the recovery range was 88%–104% and the RSD range was 1.4%–7.5% (mean of n = 6). Thus, the analytical method based on SMP yielded good results (Table 3). The LOQ and LOD were calculated based on the recovery test results. The LOQs of chlorpyrifos, diazinon, and fenobucarb were 0.002, 0.004, and 0.024 µg/m3, respectively, and the LODs were 0.0006, 0.0012, and 0.0071 µg/m3, respectively, which were well below the 1/50 guideline value (Table 3).
Based on the SMP, the analytical method for insecticides was established to be very robust, as evidenced by the calibration curve, recovery rate, LOQ, and LOD. Finally, the concentration process was evaluated (Fig. 1). The results showed that the recoveries (mean of n = 3) were 99.2% (4.4% for RSD), 87.0% (5.5% for RSD), and 81.3% (1.5% for RSD) for chlorpyrifos, diazinon, and fenobucarb, respectively. Recovery tests confirmed that the analytical results were equivalent regardless of the presence or absence of concentration. Therefore, the concentration process compensates for the poor sensitivity of the GC–MS.
Interlaboratory Validation of the Analytical Method of the InsecticidesWe developed a test method based on the SMP for insecticides, for which the guideline values for indoor air concentrations in Japan have been established. The performance of the method was further evaluated through interlaboratory validation. The ranges of accuracy (recovery rate) and precision (RSDr and RSDR) values in this test were determined by referencing the “guidelines for the validation of the testing method for drinking water.”16)
Before evaluating the results of the interlaboratory validation, we performed a Grubbs test and excluded outliers.17,18) In this test, one data set from Laboratory 5 was excluded (Table S2). In Laboratory 5, the recovery rate exceeded 151% in one of the five times, which is very high compared to the data from other institutions. We compared it to Laboratory 3, which has almost the same instrument as Laboratory 5. Since the recovery rate of Laboratory 5 varied more than that of Laboratory 3, the condition of the instrument and the deterioration of consumables such as GC columns and liners may have been factors.
The accuracies excluding outliers are shown in Fig. 2. For the case with air aspiration to the cartridges, the recovery rate ranges were 81.9%–126.3%, 76.8%–121.7%, and 76.7%–112.8% for chlorpyrifos, diazinon, and fenobucarb, respectively. In addition, the RSDr and RSDR values for each test are presented in Table 5. The target precision results were RSDr less than 30% and RSDR less than 35%. The data from all institutions ranged from 2.0% to 9.7% for RSDr and from 14.7% to 17.9% for RSDR. As a result of the interlaboratory validation, we reached a certain standard using the Grubbs test to exclude outliers. Statistical methods such as the Grubbs test may be effective in interlaboratory validation, where a certain number of outliers may occur due to differences in analytical instruments and conditions at each institution.

The Accuracy of the Interlaboratory-Validation Results for the Three Insecticides; white bar: with air aspiration, black bar: without air aspiration, error bars: the standard deviation of the recovery rate obtained at each laboratory, dashed lines: 70-130% of the target range. Air aspiration (n = 5) and without air aspiration (n = 2) were performed. Data for chlorpyrifos in Laboratory 5 was calculated, excluding outliers.

In conclusion, we have developed an analytical method for insecticides based on the SMP. The SE method employed in the SMP is easier to reanalyze than the TD method. For the SVOCs analyzed in this study, the SMP maintained sufficient sensitivity and extraction efficiency; the simplicity of the method allowed for SE advantages. Finally, the interlaboratory validation was conducted using analytical instruments from five institutions. Certain criteria were achieved by excluding outliers with the Grubbs test. We propose a method based on the SMP as the “standard test method for insecticides in indoor air” to the CIAP, and we believe that the MHLW will soon notify the public.
This study was financially supported by a Grant-in-Aid for Research on Risk of Chemical Substances (Assignment Number:21KD2002) provided by the Ministry of Health, Labour and Welfare, Japan.
Conflict of interestThe authors declare no conflict of interest.