2023 Volume 6 Issue 3 Pages 87-97
2023 Volume 6 Issue 3 Pages 87-97
Background: Blue light causes retinal photoreceptor damage via oxidative and endoplasmic reticulum (ER) stress. A previous study showed that blueberry stem extract (BStEx) and its active components have cytoprotective effects against blue-light-induced photoreceptor cell damage by suppressing oxidative stress. This study demonstrated the inhibitory effect of BStEx against blue light-induced ER stress in photoreceptor cells. Methods: The photoreceptor cells treated with BStEx or the antioxidant N-Acetyl-L-cysteine (NAC) as a positive control were used and then exposed to blue light. The cytoprotective effects of BStEx and NAC were evaluated using CCK-8. The ER stress-related protein expression changes over time, and its levels were measured after each exposure time to blue light in photoreceptor cells treated with BStEx or NAC. Results: BStEx and NAC showed protective effects against blue-light-induced photoreceptor morphological abnormalities and cell damage. Although blue light triggered ER stress factors such as BiP, PERK, ATF6, eIF2α, ATF4, and CHOP, which in turn stimulated cell cycle arrest factors p53 and p21 and upregulation of apoptosis-inducing factors caspase-3. However, BStEx suppressed the increase in expression of BiP, ATF4, ATF6, CHOP, p53, p21, and caspase-3, but not mitochondrial apoptotic factors Bax and cytochrome c. Furthermore, the antioxidant NAC showed similar suppressive effects on BStEx. Conclusion: Our findings suggest that blue light-induced ER stress is primarily caused by oxidative stress, and BStEx might suppress ER stress via an antioxidant effect. The antioxidant NAC contributes to the cell proliferative capacity and suppression of apoptosis in photoreceptor cells.
Blue light is a short-wavelength light within the visible spectrum, typically ranging from approximately 450 to 495 nm. Blue light can be found in various environmental lights, including sunlight, fluorescence, and light-emitting diodes (LED). Unlike ultraviolet light, blue light can penetrate the eye and reach the retina and macular tissue, potentially causing damage that may contribute to the development of age-related macular degeneration (AMD).1,2) In addition, a positive correlation has been observed in human studies between the onset of AMD and exposure to blue light.3) Therefore, preventing retinal cell damage from blue light exposure is important to reduce the risk of developing ocular diseases.
Endoplasmic reticulum (ER) stress is known to be one of the causes of AMD.4) ER is an intracellular organelle responsible for intracellular protein folding. The accumulation of misfolded proteins can lead to ER stress and subsequent cellular damage. The unfolded protein response (UPR), which is regulated by three transmembrane ER stress sensors: protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), promotes cell survival by maintaining protein homeostasis. However, prolonged ER stress caused by failure of the UPR to restore ER function leads to ER stress-induced apoptosis.5-7) Binding immunoglobulin protein (BiP; known as GRP78) dissociates from the sensor and activates PERK, IRE1, ATF6, eukaryotic initiation factor 2α (eIF2α) phosphorylation, which activates transcription factor 4 (ATF4) and CCAAT-enhancer-binding protein homologous protein (CHOP) expression levels, resulting in cell cycle arrest and apoptosis.8) Recent studies have reported that oxidative and ER stress are involved in the retinal photoreceptor damage induced by blue light exposure. Specifically, blue light can cause the aggregation of short-wavelength opsin (S-opsin), a photoreceptor protein, leading to ER stress.9) Studies of blue light exposure in mice have shown that S-opsin and retinal photoreceptor cells decreased in retinal tissue, and administration of the antioxidant N-Acetyl-l-cysteine (NAC) attenuated retinal lesions.10) Our previous research demonstrated that anthocyanins, resveratrol, and proanthocyanidins in bilberries and lingonberries could protect retinal photoreceptor cells from blue light-induced damage by exerting antioxidative effects.11) Additionally, bilberry extract and anthocyanins have been reported to reduce S-opsin aggregation and ATF4 activation in ER stress induced by blue light in retinal photoreceptor cells.12)
Rabbiteye blueberry (Vaccinium virgatum Aiton) is grown in Miyazaki, Japan. Their leaves and stems (Fig. 1A-C) contain various polyphenols that are different from those found in blueberry fruits. Blueberry stem hot water extract (BStEx) (Fig. 1D) is used as a food product in Japan and has been reported to inhibit lipid synthesis in the liver,13) increase anti-adult T-cell leukemia activity,14) and promote viral proliferation.15) The major polyphenols in BStEx are proanthocyanidins, composed of a polymer of cyanidin and catechin with interflavan A- and B-type linkages, and phenylpropane cinchonain I units.15) We previously showed that BStEx contains proanthocyanidins, catechins, epicatechins, chlorogenic acid, quinic acid, and caffeic acid, and it has a cytoprotective effect on retinal cell damage induced by blue-light through its antioxidant effects.16) However, it is unclear whether BStEx suppresses ER stress and whether this contributes to the protective effect against blue light-induced photoreceptor damage.

Rabbiteye Blueberry and Its Stem Extract Used in This Study and Blue LED Light Irradiation Condition for Inducing Murine Photoreceptor Cell Damage
(A) Rabbiteye blueberry (Vaccinium virgatum Aiton) grown in Miyazaki, Japan, (B) their leaves and stems, (C) cross-section of the stem, (D) blueberry stem hot water extract (BStEx) used in this study which was the same extract as in the previous our study16) and was provided by Biolabo, Co., Ltd. (E) 661W cells on a 96- or 12-well plate were placed on a transparent acrylic plate in a CO2 incubator at 37°C, and exposed from below to a blue LED light at 500 lx (shown in picture). The experimental time course shows the timepoint of each assay, such as seeding cells, culture medium replacement, cellular damage measurement, and cytolysis for western blotting.
In this study, we have demonstrated the protective effect of BStEx against blue light-induced photoreceptor cell damage and its mechanism of action involving ER stress suppression.
The BStEx used in this study was provided by Biolabo Co., Ltd. and prepared as previously reported.16) Namely, fresh stems of rabbiteye blueberries (Vaccinium virgatum Aiton; Kinisato Gou, Nanaha Corporation Co., Ltd.) were boiled in water at 98°C for 60 min. The extracted solution was freeze-dried to obtain a powder. BStEx contains 442 mg/g proanthocyanidin, 6.1 mg/g chlorogenic acid, 3.6 mg/g catechin, 4.5 mg/g epicatechin, and 78.3 mg/g quinic acid.16) N-Acetyl-l-cysteine (NAC) and dimethyl sulfoxide (DMSO) were purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan), and the protease inhibitor cocktail, phosphatase inhibitor cocktail 2, and phosphatase inhibitor cocktail 3 were purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA). Radioimmunoprecipitation Assay (RIPA) lysis buffer was purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). Blue protein loading dye was purchased from New England Biolabs (Hitchin, UK). Blocking One-P was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Can Get Signal Solution 1 and Can Get Signal Solution 2 were purchased from TOYOBO Co., Ltd. (Osaka, Japan). Clarity Max Western ECL Substrate detection reagents were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Antibodies against BiP (C50B12), PERK (C33E10), IRE1α (14C10), ATF6 (D4Z8V), phospho-eIF2α (Ser51), ATF4 (D4B8), X-box binding protein 1 (XBP1s) (E9V3E), CHOP, cleaved caspase-3, cleaved caspase-12, phospho-nuclear factor kappa B (NF-κB), NF-κB, phospho-p53 (Ser15), p53, p21 Waf1/Cip1 (12D1), anti-rabbit IgG horse-radish peroxidase (HRP)-linked antibody, and anti-mouse IgG HRP-linked antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti eIF2α (FL-315), anti-Bax (N-20), and anti-cytochrome c (H-104) antibodies were purchased from Santa Cruz Technology, Inc. (Heidelberg, Germany). The anti-β-actin antibody (clone AC-15) was purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA). The 661 W cells were a kind gift from Dr. Muayyad R. Al-Ubaidi (University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA). The blue LED irradiation devices (peak wavelength of 470 nm) were purchased from Aitec System Co., Ltd. (Kanagawa, Japan). The digital light meter model 5202 was purchased from Kyoritsu Electrical Instruments Works, Ltd. (Tokyo, Japan).
Cell CultureThe cultured photoreceptor cells (661W) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin-streptomycin-amphotericin in an incubator at 37°C in a humidified atmosphere with 5% CO2. The cells were passaged after trypsinization every two days.
Blue LED Light Irradiation of 661W Photoreceptor CellsThe irradiation of the 661W photoreceptor with blue LED light was performed following the protocol described in a previous report.16) First, the cells were seeded onto a 96- or 12-well plate with DMEM containing 10% FBS and incubated at 37°C for 24 h. After that, the medium was replaced with DMEM containing 1% FBS, and the cells were incubated at 37°C for 30 min. Next, BStEx or NAC dissolved in DMEM containing 1% FBS and 1% DMSO was added to a final concentration of 1–30 μg/mL, 0.1 and 1 mM, respectively, and the cells were incubated for 30 min. The control group consisted of cells treated with DMEM containing only 1% FBS and 1% DMSO, while the vehicle group consisted of cells irradiated with blue LED light. To perform the irradiation, 96- or 12-well plates seeded with 661W cells were placed on a transparent acrylic plate over the blue LED illumination plate, placed 300 mm below, and irradiated at 500 lx in the CO2 incubator. The control cells were shielded from exposure using aluminum foil. The temperature of the CO2 incubator was maintained at 37°C. Cell damage was evaluated 24 h after LED exposure, and cell harvest was performed at 6, 12, 24, and 36 h after LED exposure for western blotting (Fig. 1E).
Measurement of Viable Cells in Cell ProliferationFollowing 24 h of blue LED light irradiation, the culture medium was replaced with DMEM containing 1% FBS, and the viable cells were immediately assessed using CCK-8 as an indicator of cell proliferation. First, 10 μL of CCK-8 was added to each well, and the cells were incubated at 37°C for 2 h. The absorbance was measured at 450 nm using SpectraMax® ABS (Molecular Devices, LLC., San Jose, CA, USA). The 96-well plates were incubated in an incubator between measurements, and the absorbance at 0 h (blank) was subtracted from the absorbance at 2 h. Then, the percentage of undamaged cells (cytotoxicity by blue light exposure) was assessed and compared with that of the control group.
Western Blot AnalysisAfter each hour (0, 6, 12, 24, or 36 h) following blue LED light exposure, the cells were lysed in ice-cold RIPA lysis buffer containing 1% (v/v) protease inhibitor cocktail, 1% (v/v) phosphatase inhibitor cocktail 2, and 1% (v/v) phosphatase inhibitor cocktail 3. The resulting cell lysates were collected in centrifuge tubes and centrifuged at 12,000 x g for 20 min at 4°C. The supernatant was collected and mixed with blue protein loading dye [62.5 mM Tris-HCl (pH 6.8), 2% (w/v) sodium dodecyl sulfate (SDS), 10% glycerol, 0.01% (w/v) bromophenol blue, and 1.25 M dithiothreitol], and the mixture was boiled at 100 °C for 5 min. Protein samples (2–4 µg) were loaded onto a SDS-polyacrylamide gel electrophoresis (-PAGE) using an EasySeparator system (Fujifilm Wako Pure Chemical Co., Osaka, Japan). After electrophoresis, the separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes using a Wet/Tank Blotting System (Bio-Rad Laboratories, Hercules, CA, USA). The blots were blocked with Blocking One-P for 30 min at room temperature (15–25°C). Followed by probing with primary antibodies, including anti-BiP, anti-PERK, anti-IRE1α, anti-ATF6, anti-phospho-eIF2α, anti-eIF2α, anti-ATF4, anti-XBP1s, anti-CHOP, anti-cleaved caspase-3, anti-cleaved caspase-12, anti-phospho-NF-κB, anti-NF-κB, anti-phospho-p53, anti-p53, anti-p21 Waf1/Cip1, anti-Bax, anti-cytochrome c antibodies (all at a dilution of 1:1,000 in Can Get Signal Solution 1), and anti-β-actin antibody (at a dilution of 1:10,000 in Can Get Signal Solution 1) at 4°C overnight. The blots were washed thrice with Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 (0.1% T-TBS), and then probed with either anti-rabbit IgG HRP-linked antibody (1:2,000 dilution in Can Get Signal Solution 2) or anti-mouse IgG HRP-linked antibody (1:10,000 dilution in Can Get Signal Solution 2) for 60 min. After washing the blots thrice with 0.1% T-TBS, immunoreactivity was detected using Clarity Max Western ECL Substrate detection reagents. Immunoblot images were captured, and the immunoblot bands were quantified using the ImageQuant LAS 4000 system (GE Healthcare, Chicago, IL, USA).
Statistical AnalysisThe data are expressed as the mean ± SD. Statistical comparisons were performed using analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test. The threshold for statistical significance was set at p < 0.05.
We investigated the protective effects of BStEx and NAC (used as a positive control for its antioxidant properties) at various concentrations against blue light-induced photoreceptor cell damage (Fig. 2). After 24 h of blue LED light exposure, the cells exhibited morphological changes such as decreased cell number and increased spherical cells, leading to apoptosis, which was observed microscopically in the vehicle (Fig. 2A). However, treatment with 30 μg/mL BStEx and 1 mM NAC prevented the morphological alterations. In addition, morphological changes in cell shape were not observed after treatment with BStEx and NAC without blue light exposure.
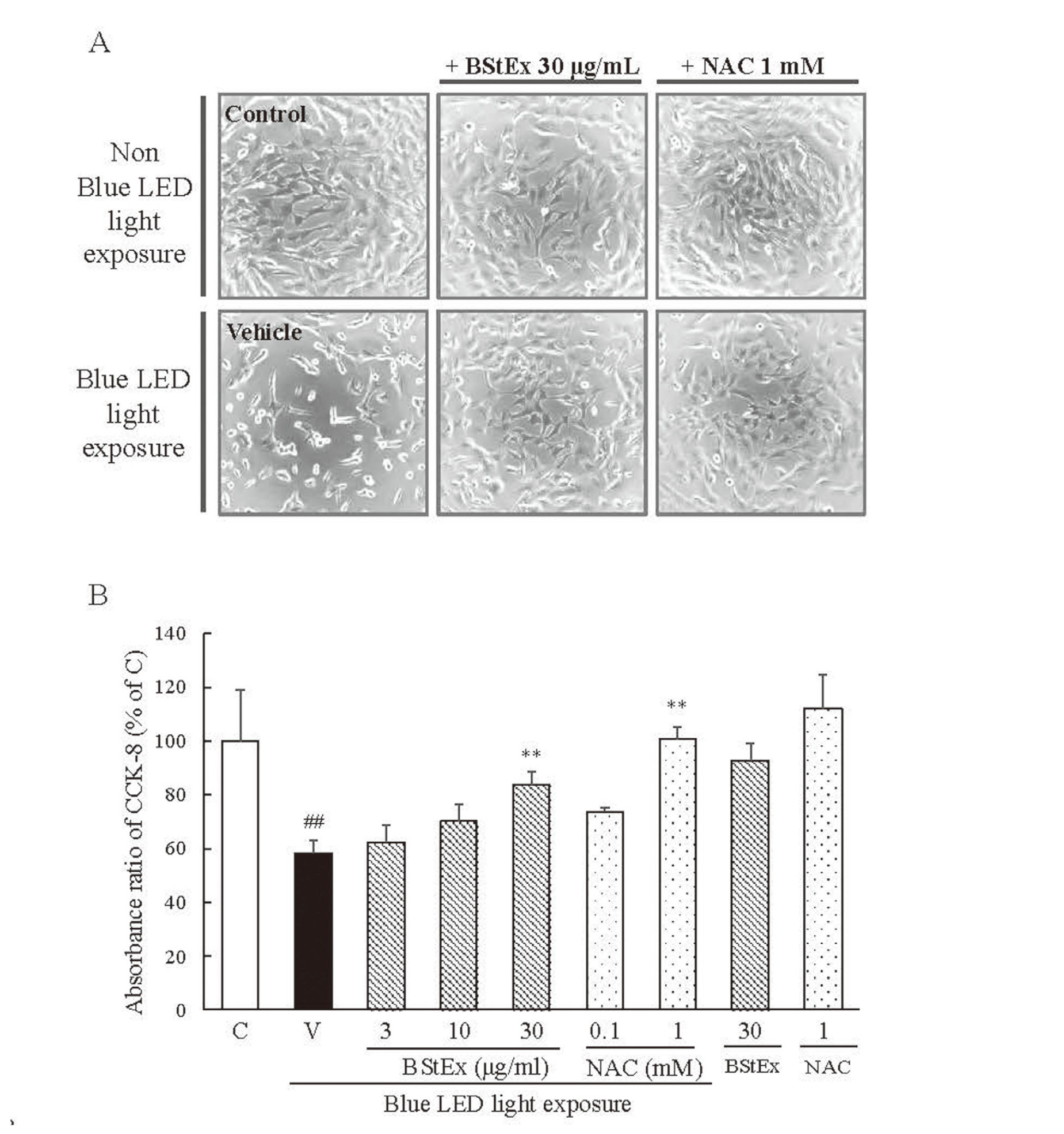
The Protective Effects of BStEx and NAC Against the Cell Morphology Alteration and Damage Induced by Blue Light in 661W Cells
(A) The 661W cell morphology was observed after treatment with BStEx and NAC in blue light irradiation. (B) BStEx and NAC prevent the blue LED light-induced reduction of CCK-8 absorbance, indicating cellular damage after 24 h of blue LED light exposure. Data are represented as the mean ± SD (n = 3). C, control; V, vehicle; BStEx, blueberry stem extract; NAC, N-acetyl-l-cysteine; Fr., fraction; eq., BStEx 30 µg/mL equivalent dose. #p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. the vehicle-treated group (Tukey–Kramer multiple comparison test)
After blue LED light exposure for 24 h, the absorbance of CCK-8 was reduced, indicating a decrease in the number of undamaged photoreceptor cells. However, treatment with 30 μg/mL BStEx or 1 mM NAC significantly increased the absorbance after 24 h of blue light exposure to 661W cells (Fig. 2B). Furthermore, incubation of photoreceptor cells with BStEx (30 μg/mL) and NAC (1 mM) in the absence of blue light exposure for 24 h did not induce cytotoxicity. These findings demonstrate that BStEx and NAC have cytoprotective effects against blue light exposure.
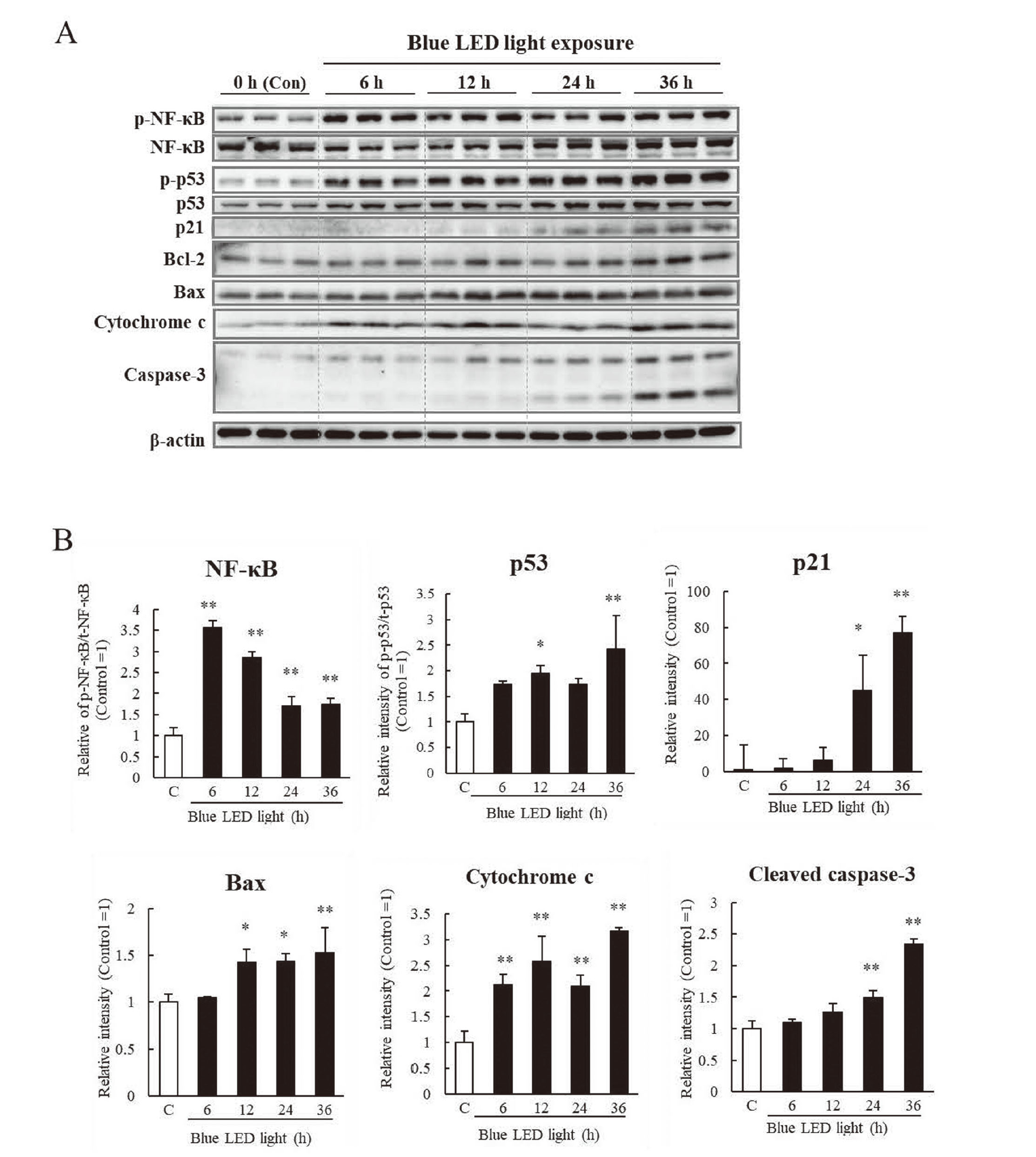
Blue Light Causes ER Stress in Photoreceptor Cells Over TimeATF4 activation during ER stress contributes to blue light exposure in retinal photoreceptor cells.12) In this study, to clarify changes over time in ER stress-related factors under blue light exposure conditions, we examined the expression of ER stress-related proteins in photoreceptor cells at 6, 12, 24, and 36 h after blue light exposure, based on the control (0 h without blue light exposure). The expression of BiP, PERK, eIF2α, ATF4, ATF6, CHOP, IRE1α, and XBP1s were increased by blue light, except for cleaved caspase-12 (Fig. 3A). BiP expression was highest at 6 h of blue light exposure. However, it decreased to a similar level as the baseline expression after 24 h. In response to the increase in BiP expression, PERK, ATF6, and IRE1α expression were increased after 6 h. Significant increases were observed after 6 h for ATF6, 12 h for IRE1α, and 24 h for PERK. The activation of eIF2α and expression of ATF4, which are downstream of PERK, significantly increased from 6 h of blue light exposure and continued to increase until 36 h. The expression of CHOP, which is involved in caspase-3-related apoptosis owing to the upregulation of ATF6 and ATF4, increased on blue light exposure and peaked at 24 h. In contrast, XBP1s downstream of IRE1α were upregulated after 36 h of blue light exposure. Caspase-12 is specifically cleaved in cells undergoing ER stress-induced apoptosis. Caspase-12, which is specifically cleaved in cells undergoing ER stress-induced apoptosis, expression tended to increase; the increase was insignificant. This suggests that caspase-12 might not be involved in cell death induced by blue light irradiation. These results indicated that blue light irradiation increased CHOP expression via the ATF4 and ATF6 pathways due to increased BiP over time.

Blue Light-Induced Changes in ER Stress-Related Proteins in Photoreceptor Cells Over Time
(A) Band images show immunoreactivity of ER stress-related proteins by western blotting at 0 (as control), 6, 12, 24, and 36 h of blue light exposure. (B) Quantitative analysis of protein based on control. Data are represented as the mean ± SD (n = 3). C, control. #p < 0.05, ##p < 0.01 vs. control (Tukey–Kramer multiple comparison test)
We conducted further investigations on the expression changes of the cell cycle arrest pathways, such as NF-κB, p53, and p21, and mitochondrial apoptosis-related factors, such as Bax, cytochrome c, and cleaved caspase-3, over time, at the point of 0 h (as controls), 6, 12, 24, and 36 h after blue light exposure. Phosphorylated NF-κB expression was highest after 6 h of blue light exposure and decreased at 24 h. Activation of NF-κB was followed by increased expressions of p53 and p21 at 12 h and 24 h, respectively (Fig. 4). In addition, Bax and cytochrome c expressions increased after 12 h and 6 h, respectively. Moreover, cleaved caspase-3 expression increased from 24 h to 36 h, which could be attributed to the increased expression of Bax, cytochrome c, or CHOP increased by blue light.
Blue Light-Induced Changes in Cell Cycle Arrest and Mitochondrial Apoptosis-Related Proteins in Photoreceptor Cells Over Time
(A) Band images show immunoreactivity of cell cycle arrest and mitochondrial apoptosis-related proteins by western blotting at 0 (as control), 6, 12, 24, and 36 h of blue light exposure. (B) Quantitative analysis of protein based on Control. Data are represented as the mean ± SD (n = 3). C, control. #p < 0.05, ##p < 0.01 vs. control (Tukey–Kramer multiple comparison test)
The effects of BStEx or NAC on the expression of ER stress-related proteins were examined using western blotting in photoreceptor cells treated with BStEx (Fig. 5A) or NAC (Fig. 5B) during blue light irradiation at time points where the expression of ER stress-related factors was upregulated, as shown in Fig. 3. BStEx treatment significantly suppressed the increase in BiP expression in blue light-exposed photoreceptor cells (Fig. 5C). In contrast, the phosphorylation of eIF2α and the expression of ATF4 downstream of the PERK pathway were upregulated by blue light, and BStEx significantly suppressed only ATF4. BStEx treatment suppressed the upregulation of IRE1α and ATF6 and contributed to the suppression of CHOP and cleaved caspase-3 expression. The antioxidant NAC significantly suppressed the expression levels of BiP, ATF4, ATF6, CHOP, and their induced cleavage by caspase-3 and inhibited eIF2α phosphorylation (Fig. 5D). These results indicate that BStEx might have suppressed the upregulation of CHOP via the BiP to ATF4 pathway and caspase-3, similar to the antioxidant NAC. BStEx and NAC treatments did not affect the expression of XBP1s. Figs. 5A and 5B show the western blotting bands but were not quantified.
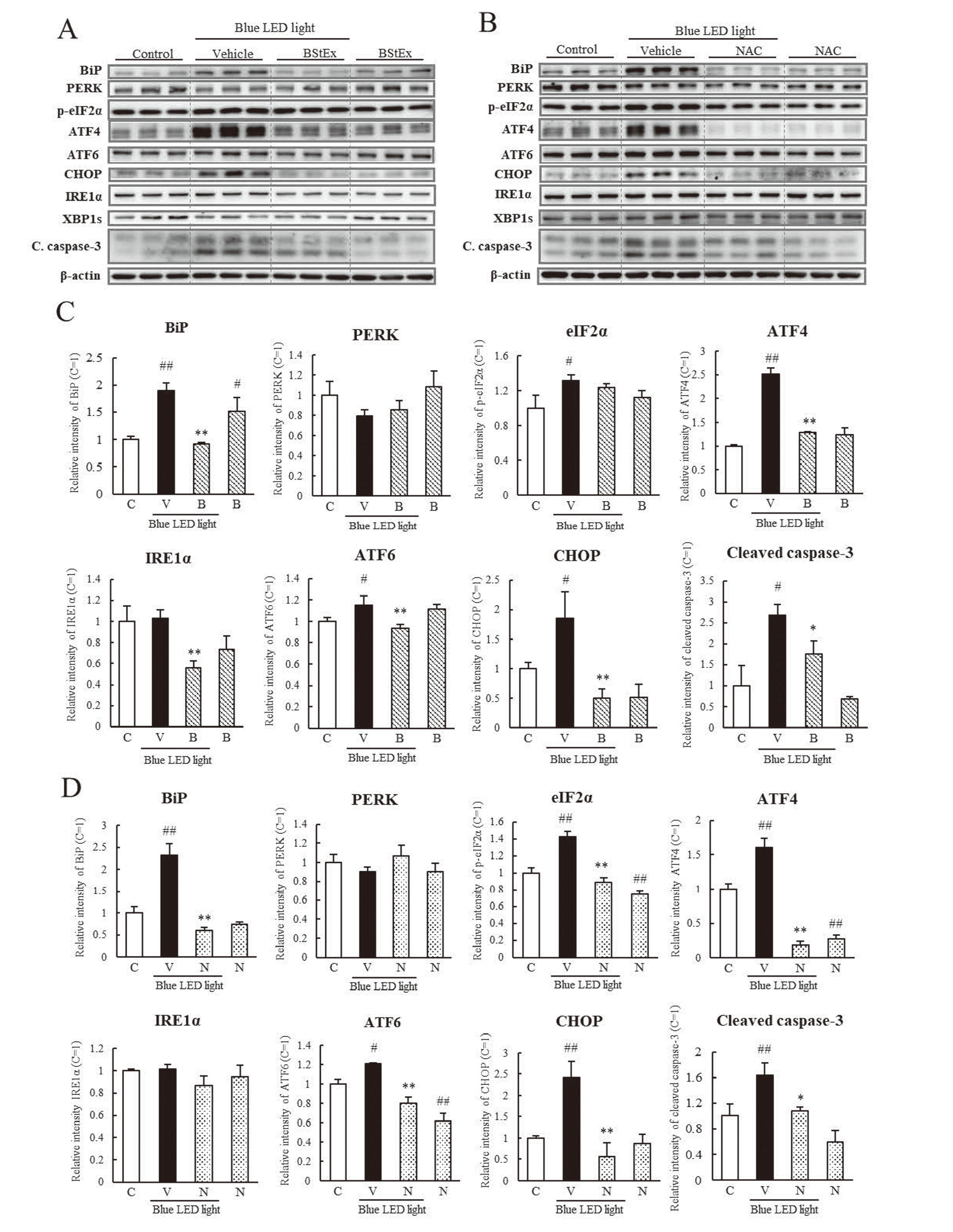
The Effects of BStEx and NAC Against Blue LED Light-Induced Endoplasmic Reticulum (ER) Stress and Apoptosis-Related Proteins on Western Blot Analysis
Band images show immunoreactivities proteins by treatment with (A) BStEx and (B) NAC. Quantitative analysis of proteins by (C) BStEx and (D) NAC treatment shows band densities as a percentage of control. Data are represented as the mean ± SD (n = 3). C, control; V, vehicle; B and BStEx, blueberry stem extract; N and NAC, N-acetyl-l-cysteine. #p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. the vehicle-treated group (Tukey–Kramer multiple comparison test)
The shape, number, and CCK-8 levels of photoreceptor cells decreased after blue light irradiation (Fig. 2), implying a reduction in cell proliferative capacity. Previous studies have shown that oxidative and ER stress affects cell cycle arrest. Therefore, we investigated the factors related to cell cycle arrest using western blotting (Fig. 6A, B). BStEx and NAC significantly suppressed the phosphorylation of NF-κB and increased the expression of p53 and p21 induced by blue light (Figs. 6C and 6D). However, the expression of mitochondrial apoptosis-related proteins, Bax and cytochrome c, was not increased by blue light irradiation at the same time points compared to the control. BStEx and NAC treatments did not suppress their expression but increased it under non-blue light conditions. These results suggest that BStEx and NAC affect the stability of proliferative capacity by suppressing cell cycle arrest-related protein expression induced by blue light irradiation; they may not contribute to the suppression of mitochondrial apoptosis.
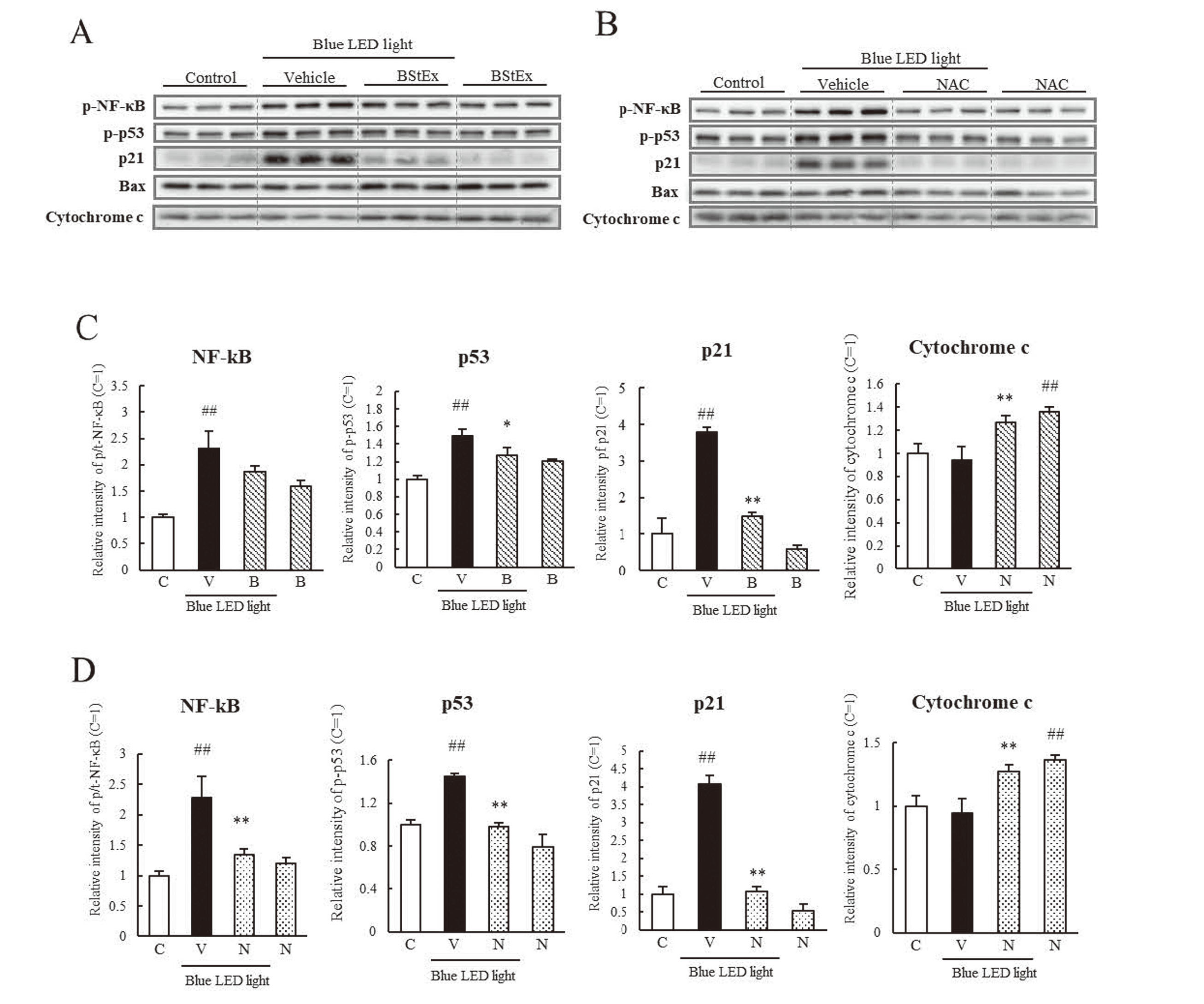
The Effects of BStEx and NAC Against Blue LED Light-Induced Cell Cycle Arrest and Mitochondria-Related Proteins on Western Blot Analysis
Band images show immunoreactivities of proteins by treatment with (A) BStEx and (B) NAC. Quantitative analysis of proteins by (C) BStEx and (D) NAC treatment shows band densities as a percentage of control. Data are represented as the mean ± SD (n = 3). C, control; V, vehicle; B and BStEx, blueberry stem extract; N and NAC, N-acetyl-l-cysteine. #p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. the vehicle-treated group (Tukey–Kramer multiple comparison test)
Blue light is a short-wavelength visible light with high energy that can reach the retina in the posterior eye and cause cellular damage. We previously reported the protective effects of BStEx and the antioxidant NAC as positive controls against blue LED light-induced photoreceptor cell damage via an antioxidative effect. Recently, it has been reported that light induces oxidative and ER stress and the aggregation of S-opsin in retinal photoreceptors.9,17) Therefore, we examined the cytoprotective effects of BStEx to determine whether these effects are mediated by the suppression of ER stress caused by blue light.
We investigated the cytoprotective effects of BStEx and NAC against photoreceptor cell damage induced by blue light irradiation. In our previous study, we observed that the apoptosis of cells after 24 h of blue light exposure was as low as 8%, which could be in the early apoptotic stage; the number of apoptotic cells was reduced by BStEx and NAC treatments.16) BStEx and NAC improved the blue light-induced cell morphology abnormality; round-shaped cells probably induced apoptosis, reduced the number of cells by microscopic observations, and ameliorated CCK-8 absorbance in a concentration-dependent manner, suggesting preventive effects on the reduction of cell proliferation after 24 h of blue light exposure (Fig. 2).
We examined the activation of ER stress factors, the BiP/PERK/eIF2α/ATF4 signaling pathway, the IRE1α/XBP1s signaling pathway, ATF6, CHOP, and caspase-12 upon blue light exposure (Fig. 3). We observed that the expression of BiP increased at 6 h of blue light exposure, indicating the induction of ER stress in the early stage of blue light exposure. The upregulation of BiP was followed by a significant upregulation of eIF2α and ATF4 downstream of PERK and ATF6 activation from 6 to 12 h. The activation of ATF4 and ATF6 increased CHOP expression from 12 to 24 h, accompanied by a significant increase in caspase-3 at 24 h (Fig. 4). IRE1α was significantly upregulated after 12 h of blue light irradiation. However, XBP1s, a downstream factor of IRE1α, was significantly upregulated after 36 h, indicating that IRE1α may not affect caspase-3 induction (Fig. 3B). Caspase-12 is produced in the ER via an increase in Ca2+ ;18) however, its expression was not significantly upregulated by blue light in this study, indicating it is unlikely to be involved in the induction of apoptosis (Fig. 3B). These ER stress response flows were demonstrated by the upregulation time. Additionally, we observed an increased expression of Bax and cytochrome c upon blue light irradiation (Fig. 4B). The blue light induces mitochondrial damage and apoptosis,19) suggesting that mitochondrial apoptosis is associated with ER stress involved in blue light-induced photoreceptor apoptosis. Based on these findings, we investigated the mechanism of the cytoprotective effect of BStEx against blue light-induced photoreceptor cell alteration.
We thoroughly investigated the inhibitory effects of BStEx and the positive control antioxidant NAC on the upregulation of each ER stress-related factor. Our results demonstrated that BStEx has an inhibitory effect like NAC's (Fig. 5). BStEx appeared to inhibit apoptosis by suppressing the activation of caspase-3 via BiP, ATF4, ATF6, and CHOP-mediated activation of ER stress-related proteins (Fig. 6). In contrast, NAC significantly inhibited eIF2α phosphorylation, whereas BStEx showed no inhibitory effect on its phosphorylation. However, BStEx significantly reduced the elevated expression of its downstream ATF4 (Figs. 5C and 5D). Previous studies have reported that cyanidin, a polyphenol, showed an ATF4-specific inhibitory effect on ER stress induced by blue light in RPE cells.20) Additionally, proanthocyanidin derived from grape seed attenuated ATF4 in the liver in an ischemia/reperfusion injury mouse model.21) These previous reports indicate that BStEx involving proanthocyanidins may have an inhibitory effect on the activation of ATF4 by blue light. The results of the ATF4 reduction might contribute to the inhibition of CHOP and caspase-3 upregulation. BStEx demonstrated downregulation of IRE1α expression under blue light exposure, unlike NAC; however, the mechanisms were unclear in this study. The downregulation of IRE1α did not affect the expression of its downstream XBP1s, indicating that the downregulation of IRE1α by BStEx might not be associated with the cytoprotective effects (Fig. 5A). Based on these findings, we hypothesized that BStEx suppressed blue light-induced ER stress in photoreceptor cells through a mechanism similar to that of the antioxidant NAC. Thus, BStEx may have specifically inhibited ATF4 activation by blue light.
Blue light irradiation induces mitochondrial damage, which accelerates oxidative stress and induces mitochondrial apoptosis.22) In this study, we observed increased expression of Bax and cytochrome c (Fig. 4); thus, we examined whether BStEx and NAC contributed to the inhibition of the mitochondrial apoptotic pathway. Our results showed that BStEx and NAC treatment significantly upregulated Bax and cytochrome c expression (Figs. 6C and 6D). However, BStEx and NAC treatment reduced blue light-induced cytotoxicity, suggesting that the increased expression of Bax and cytochrome c by BStEx and NAC might not contribute to apoptosis. Thus, BStEx and NAC may not have an inhibitory effect on blue light-induced mitochondrial apoptotic responses.
The reduction in the number of photoreceptor cells due to blue light irradiation was observed, as shown in Fig. 2. We investigated the influence of blue light irradiation on the cell proliferative capacity. p53 is a well-known DNA damage response protein that is activated in the retinal photoreceptor and RPE upon light irradiation.23) p53 activation causes cell cycle arrest at the G1 and S phases via p21, a cyclin-dependent kinase inhibitor, which regulates and delays cell proliferation. p53 activation is triggered by NF-κB.24) NF-κB and p53 activation were upregulated after 6–12 h of blue light exposure (Fig. 4B). We observed that BStEx and NAC treatment improved the cell number after 24 h of blue light irradiation, as shown in the absorbance results of CCK-8 (Fig. 2B). We examined the expression of p53, p21, and NF-κB in photoreceptor cells treated with BStEx and NAC by western blotting. We observed that the increase in p53 and p21 expression upon exposure to blue light was significantly suppressed by BStEx and NAC treatment (Figs. 6C and 6D). Additionally, NAC significantly suppressed NF-κB activation, while BStEx showed a suppressive trend but not a significant decrease. ROS production is a known cause of p53 activation,23) and BStEx suppressed p53 through its antioxidant effect. p21 has been reported to be mediated by CHOP.8) Accordingly, BStEx and NAC act via CHOP suppression in ER stress to inhibit the upregulation of p21 expression and thus might prevent cell cycle arrest.
BStEx contains a variety of low and high degrees of polymerization of proanthocyanidins. However, the structure of the proanthocyanidins in BStEx has not yet been determined.15) Previous studies have suggested that the degree of polymerization of polyphenols affects their antioxidant activity, with proanthocyanidins having B-type linkages and a degree of polymerization around 10 having a higher anion radical scavenging capacity.25) In contrast, the effect of proanthocyanidins on lipid oxidation is associated with a lower degree of polymerization and a higher inhibitory effect on lipid peroxidation.26) In addition, BStEx contains a complex of polyphenols with antioxidant properties, such as chlorogenic acid and catechins, which have been reported to inhibit intracellular ROS production by blue light in our previous study.16) Therefore, proanthocyanidins and polyphenols in BStEx contributed to the suppression of ER stress via antioxidant effects similar to those of NAC. However, this study did not investigate the components of BStEx, such as proanthocyanidins or other polyphenols, which contribute to the inhibition of blue light-induced ER stress. Thus, identifying the active components of BStEx that lead to ER stress suppression is an important topic for future research.
Proanthocyanidins are known to have low absorption following oral intake. However, recent reports have shown that intestinal bacteria degrade proanthocyanidin polymers and absorb them as monomers or dimers27). In contrast, proanthocyanidin dimers, cyanidins, and catechins may be absorbed into the bloodstream and exert their effects. Catechins and chlorogenic acid in BStEx may be available in low blood levels via oral consumption.28, 29) Thus, the polyphenolic components in BStEx may have a combined effect in vivo. However, future studies must examine the blood and eye metabolism of the active ingredients, mainly BStEx and proanthocyanidin polymers, after oral administration. Additionally, it would be useful to study the protective effect against blue LED light photoreceptor cell damage first by intraocular injections and then oral intake in vivo.
In conclusion, our study demonstrated that blue light-induced ER stress in photoreceptor cells activates the BiP/PERK/eIF2α/ATF4 and ATF6 signaling pathways from the early stage of exposure, leading to CHOP/caspase-3 apoptosis, simultaneous cell cycle arrest, and impaired cell proliferative capability (Fig. 7). BStEx treatment suppressed ER stress responses, particularly the CHOP/caspase-3 apoptosis pathways mediated by ATF4 signaling and p53/p21 cell cycle arrest by blue light via an antioxidative effect similar to NAC.
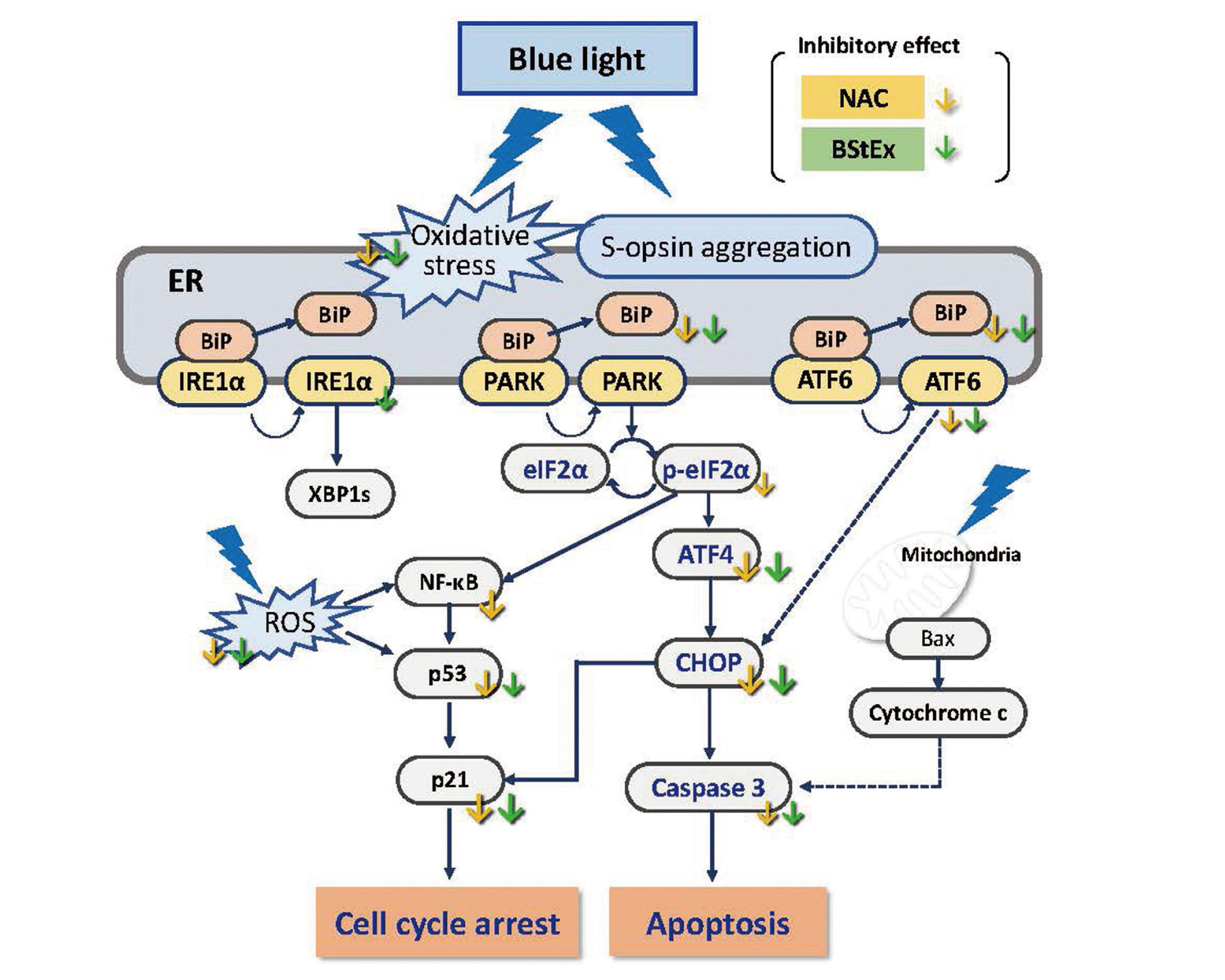
The Summary Mechanism of the Effect of BStEx and Antioxidant NAC Against Blue Light-Induced ER Stress, Cell Cycle Arrest, and Apoptosis in Photoreceptor Cells
Blue light induces ER stress by oxidative stress and S-opsin aggregation, and BStEx and NAC suppressed the increase in BiP, which triggers ER stress, and consequently suppressed ATF6. In contrast, NAC suppressed eIF2α phosphorylation in the PERK pathway, which is mainly activated by blue light, but BStEx treatment did not suppress it. However, BStEx may have inhibited caspase-3 activation via downstream CHOP by suppressing ATF4. Although ROS produced by blue light-induced activation of NF-kB and p53, the antioxidant NAC suppressed them, while BStEx treatment tended to suppress NF-κB, and significantly inhibited p53. The cell cycle arrest factor p21 was significantly suppressed by BStEx and NAC treatment, possibly via suppression of p53 and ER stress-related CHOP. In contrast, BStEx and NAC had no effect on Bax and cytochrome c, which are mitochondrial apoptotic factors induced by blue light. Our results suggest that ER stress-mediated by ATF4 and CHOP induced by blue light is involved in cell cycle arrest response and apoptosis induction. In addition, BStEx reduced oxidative stress like NAC and may have a potent inhibitory effect on ATF4 induced by blue light. Green and yellow arrows indicate the inhibitory effects of BStEx and NAC treatments, respectively.
The authors thank the Institute for Tenure Track Promotion, University of Miyazaki, for supporting this research. In addition, the authors thank Jun-ichi Hikima, Graduate School of Agriculture, University of Miyazaki, for providing cell observation techniques. A part of this work was conducted at the Frontier Science Research Center, University of Miyazaki. We want to thank Editage (www.editage.com) for the English language editing.
Conflicts of interestY. G. and S. N. are employees of Biolabo Co., Ltd., which provided the blueberry stem extract.