2023 Volume 6 Issue 3 Pages 98-102
2023 Volume 6 Issue 3 Pages 98-102
Amiodarone and tramadol are known to have drug-drug interactions that potentiate the effects of the anticoagulant warfarin. Herein we report a case which suggests increasing prothrombin time-international normalized ratio (PT-INR) and PT-INR/dose ratio due to the concomitant administration of warfarin, amiodarone (a cytochrome P450 2C9 inhibitor) and tramadol (an enhancer of anticoagulant effects of warfarin). A 72-year-old woman underwent emergency surgery following a suspected non-obstructive mesenteric ischemia, and diagnosis was confirmed. After surgery, the patient was given 2 mg/day of WF, and tramadol/acetaminophen, followed by amiodarone. After the concomitant dose of the triple combination, the PT-INR increased to over 7.02 (above the upper limit of the laboratory). The physician consulted the pharmacist for dose adjustment, and the pharmacist recommended a reduction in the dose to 0.5 mg. After restarting warfarin, PT-INR was controlled to 1.66–2.02. However, the PT-INR/dose ratio increased from 1.22 to 3.32–4.04 compared to the initial dose. The results suggest that these enhanced anticoagulant effects may be due to the inhibition of WF metabolism. Although the patient underwent resection of the small intestine, the effects of oral vitamin K1 were observed one day after administration. In conclusion, frequent PT-INR monitoring, and pharmacist intervention such as the assessment and dose adjustment in this report should be beneficial during anticoagulation therapy when multiple concomitant medications with suspected drug-drug interactions are present.
The anticoagulant warfarin (WF) is one of the most commonly prescribed oral anticoagulants. WF comprises a racemic mixture of S-WF and R-WF, mainly metabolized by cytochrome P450 (CYP) 2C9 and 3A4, respectively.1) Since WF is a vitamin K antagonist, foods and herbal medicines containing vitamin K act antagonistically, with many known interactions in metabolic pathways and pharmacological action points.2,3) Amiodarone, an antiarrhythmic drug, is metabolized by CYP in the liver. It undergoes biliary excretion and is also known to inhibit CYP isoenzymes 1A2, 2C9, 2D6, 3A4, 3A5, and 3A7.4,5) Owing to these metabolic properties, amiodarone alters the anticoagulation markers such as increasing prothrombin time (PT) or international normalized ratio (INR) of patients on stable dosages of WF.4-8) Although the mechanism of action is unknown, tramadol also has a drug-drug interaction (DDIs) with WF, leading to prolonged PT-INR.9-11) These reports are for single-agent combinations with WF, with no reports of multi-drug administration in combination with WF, amiodarone, and tramadol. Tighter PT-INR control is considered important in patients on hemodialysis because the meta-analysis reported that WF increased the risk of major bleeding in patients with atrial fibrillation and end-stage chronic kidney disease, including those on renal replacement therapy.12)
Here, we report a case of WF DDI with amiodarone and tramadol/acetaminophen. The patient underwent emergency surgery for a partial small intestine resection and colostomy with a diagnosis of nonocclusive mesenteric ischemia. A PT-INR/dose of WF ratio13) was used to rule out dose-dependent changes in anticoagulant effects and to confirm effects on metabolic processes. This work was approved the University of the Ryukyus Ethics Review Committee (Approval No. 1888).
A 72-year-old woman (height 145.8 cm, body weight 46.1 kg) with type 1 diabetes, diabetic nephropathy, ischemic heart disease (after percutaneous coronary intervention and coronary artery bypass graft), low left heart function, congestive heart failure, and Hashimoto's disease was on maintenance dialysis. She was admitted to the hospital for shunt failure, but had intermittent epigastric mild abdominal pain and vomiting for 2–3 days before admission. The patient underwent emergency surgery for partial resection of the small intestine and colostomy, on day 3 of hospitalization, for suspected diagnosing non-obstructive mesenteric ischemia and diagnosed. Anticoagulation with heparin was initiated at 10,000 U/day from day –1 of WF treatment on failure to aspirate blood from the catheter lumens during hemodialysis, which was changed to 2 mg/day WF taken after every dinner (18:30) on day 1 of WF treatment, with a target PT-INR of 1.6–2.0. To treat persistent right foot pain, tramadol/acetaminophen combination tablets (37.5 mg/325 mg) were administered: one tablet twice daily and alprostadil 10 μg/day on the same day. The PT-INR before WF initiation was 1.48 (Table 1). Blood samples were collected between 6:00 and 7:00 am every morning. On day 2 of WF treatment, paroxysmal atrial fibrillation (pAF) developed, and amiodarone was initiated at 100 mg/day. Other drugs were used concomitantly: bisoprolol fumarate 1.25 mg/day, furosemide 160 mg/day, nicorandil 15 mg/day, atorvastatin 10 mg/day, esomeprazole 20 mg/day, levothyroxine sodium 75 μg/day, aspirin 100 mg/day, and Daikenchuto 15 g/day. On day 5 of WF treatment, PT-INR increased to > 7.02 (above the upper laboratory limit), and WF was discontinued. On day 7 of WF treatment, PT-INR remained elevated and persistent at > 7.02, and the patient was given 5 mg of vitamin K1 orally. During this period, the physician consulted with the pharmacist to discuss WF interactions and was advised that a drug interaction between WF and amiodarone was possible and that the WF dose should be reduced when resumed or used in combination. PT-INR decreased to 1.86 on day 12 of WF treatment, and the physician consulted with the pharmacist again to determine the restart dose. The pharmacist suggested restarting WF treatment at 0.5 mg/day, and this recommendation was accepted. After restarting WF, PT-INR was 1.66 on day 14, 1.75 on day 15, and 2.02 on day 18 (Fig. 1). PT-INR/dose during WF treatment was 1.22 before concomitant administration of amiodarone but increased to 3.32–4.04 after restart of WF (Fig. 2). The PT-INR/dose ratios are defined as the WF per dose one day before blood sampling. The patients had no abnormal observations of liver function; aspartate aminotransferase and alanine aminotransferase on days 1, 5, and 12 of WF treatment were 26 and 10, 26 and 8, and 14 and 8 (U/L), respectively. When the patient underwent emergency surgery for suspected non-obstructive mesenteric ischemia, the same time, meropenem was started for the treatment of intestinal infection, and vancomycin was added later. Antibiotic treatment had discontinued when WF was initiated.
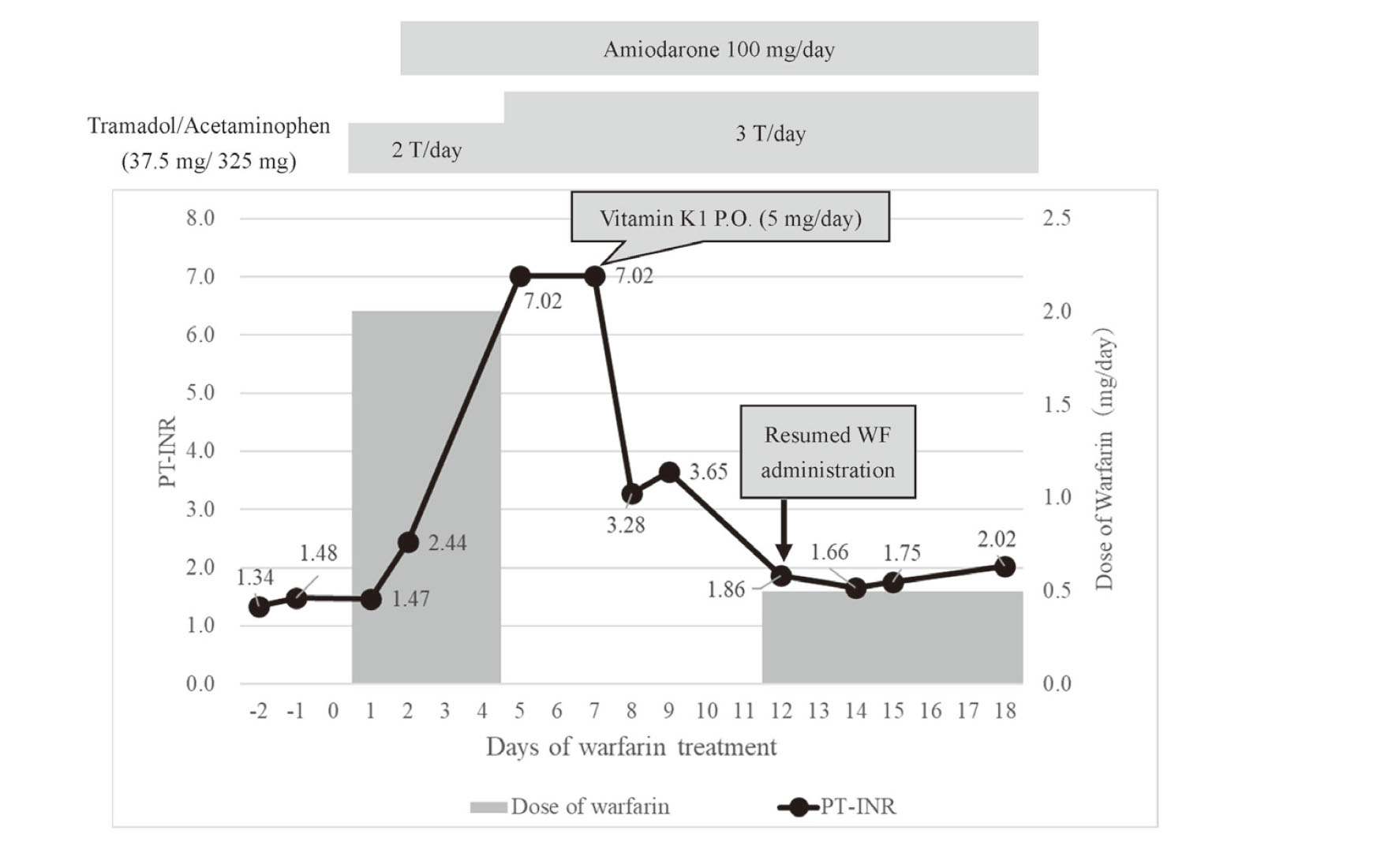
Change in prothrombin time-international normalized ratio (PT-INR) and warfarin dosage (mg/day) in the patient. P.O.: Per Obs.
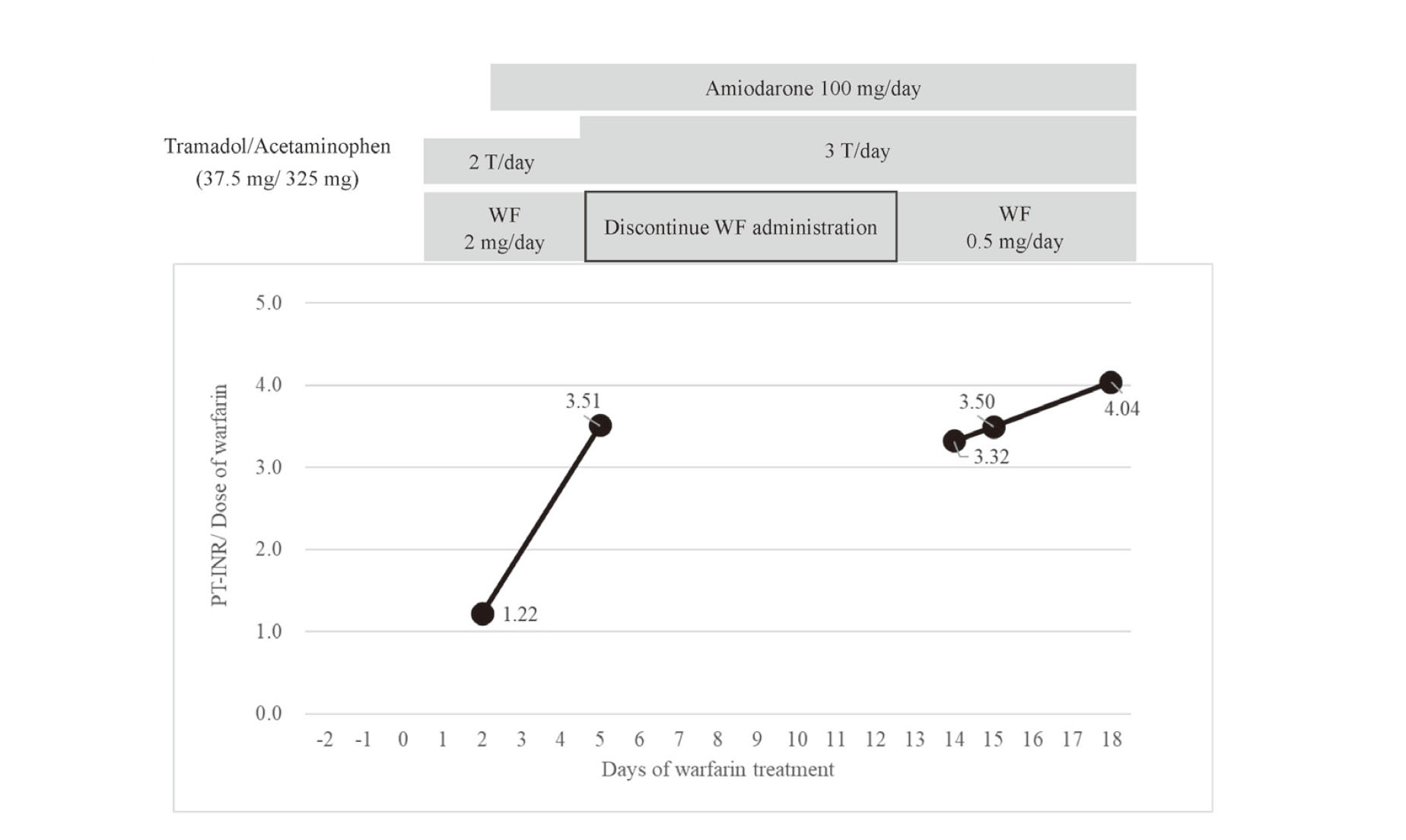
Change in prothrombin time-international normalized ratio (PT-INR)/daily dose of warfarin (mg/day) in the patient.
In this case, based on the concomitant medications, drug interactions due to amiodarone inhibition of CYP3A4 and CYP2C9, and tramadol-enhanced anticoagulation were believed to cause the elevated PT-INR. Amiodarone enhances the anticoagulant effects by inhibiting CYP2C9 and 3A4, the major metabolic enzymes of WF, and by reducing the metabolic clearance of both S-WF and R-WF.14) Almog et al. reported a significant correlation between the dose reduction of WF with that of amiodarone (mg/ kg · day) when they were combined.6) The starting dose of amiodarone (2.2 mg/kg) administered to the patient in their study required an estimated WF dose reduction of 25%; however, the patient's PT-INR was ultimately adjusted to approximately 2.0 with a 0.5 mg maintenance dose of WF. As a result, after concomitant administration with amiodarone and tramadol/acetaminophen, the WF dose had to be reduced by 75% of the starting dose. Naganuma et al. reported that the plasma concentration of desethylamiodarone, which is a metabolite of amiodarone but not amiodarone, correlated with fluctuations in WF PT-INR/dose and might be involved in CYP2C9 inhibition, and that the plasma concentration of desethylamiodarone increased after 7 days starting of amiodarone treatment, with little accumulation before that time.15) These data suggest that the increase in PT-INR on day 5 in this patient was likely to have been induced by desethylamiodarone. In the present case, a tramadol hydrochloride (37.5 mg) / acetaminophen (325 mg) combination tablet was co-administered with WF and amiodarone. Sabbe et al. reported that the concomitant use of tramadol 50 mg every 6 h in a 61-year-old patient on WF 5 mg (Monday, Wednesday, Friday) and 7.5 mg (Tuesday, Thursday, Saturday, and Sunday), increased PT- INR from a therapeutic range (2.5–3.5) to 10.6 within three days of initiation.9) Dumo et al. also reported an increase in PT-INR from 2.5 to 6.14 over nine days with tramadol 50 mg twice/day in a 65-year-old patient on WF 8 mg (Tuesday, Thursday, and Saturday), and 9 mg (Monday, Wednesday, Friday, and Sunday).10) Tramadol is metabolized by CYP2D6 and CYP3A4, and a possible CY3A4-mediated interaction with WF was noted.10) In the present case, the co-administration of amiodarone and tramadol may have increased PT-INR on day 5 of WF treatment and lower WF maintenance dose after resumption, compared with that reported for amiodarone alone.6) In addition, a marked increase in PT-INR/dose was observed, suggesting that these enhanced anticoagulant effects may be due to inhibition of WF metabolism. Therapeutic drug monitoring (TDM) is recommended for amiodarone to confirm its efficacy and avoid adverse events.16) However, blood levels of amiodarone were not evaluated in the present case. Although evaluating blood levels of drugs that may induce interactions is helpful for predicting drug interactions, the significant increase in PT-INR on day 3 after administering a combination of WF and amiodarone observed in the patient highlights the potential increase in risk even in the short term before TDM is performed. In addition, the mean half-life of amiodarone is 40–55 days (range 28–107 days).17) Therefore, it may take 130–535 days (five half-lives) for amiodarone to disappear. Consequently, the effects of interaction of amiodarone with WF will persist for an extended period even after their discontinuation.
In this case, because tramadol was used in addition to amiodarone, it is reasonable to evaluate PT-INR frequently after concomitant use of WF and multiple drugs suspected to interact, and to reduce or discontinue WF with possible administration of an antagonist (vitamin K), if an increase in PT-INR is observed. Studies on the gastrointestinal absorption of WF have shown that WF is potentially absorbed in the region from the stomach to the duodenum.18-20) The patient in the present study underwent a partial resection of the small intestine due to non-obstructive mesenteric ischemia, and there was concern regarding the reducing effect of the drug on gastrointestinal absorption. However, PT-INR was managed at a final dose of WF 0.5 mg, indicating that the effect of partial resection might be minimal.
Vitamin K, a WF antagonist, has been used in over-warfarinized patients21) and has also been reported to be absorbed via the Niemann-Pick C1-like 1 (NPC1L1) transporter localized in the small intestine.22) In the patient in this study, a rapid decrease in PT-INR from >7.2 on day 7 to 3.28 on day 8 was observed following oral administration of vitamin K1. Pendry et al. reported that in 30 patients with over-warfarinization (INR>8.0), 40% of patients had an INR <4.5 within 24 h after receiving 1 mg of oral vitamin K.23) Watson et al. also reported that 50% (n=6, median INR: 4.1 to 8.2) of WF-treated patients with INR>5 achieved an INR<4.0 at 24 h after 1 mg of oral administration of vitamin K, and 60% (n=18, median INR: 4.2 to 9.3) of WF-treated patients with INR>5 achieved an INR<4.0 at 24 h following administration of 5 mg of vitamin K.24) In the present case, a PT-INR of <4.0 was achieved the day after oral vitamin K1 was administered. Therefore, if PT-INR is elevated because of a DDI, it may be possible to prevent risk of bleeding by immediately discontinuing WF and promptly administering vitamin K. It was impossible to confirm the exact area resected from the small intestine from the medical record; the lower small intestinal area was attributed to the colostomy. In the postoperative state described above, and amidst the residual effects of amiodarone-mediated inhibition of WF metabolism and tramadol-mediated enhancement of anticoagulation, the rapid decrease in PT-INR after vitamin K administration suggests that the effect on vitamin K absorption was negligible.
The patient had received a peripheral intravenous nutritious transfusion (BFLUID® Injection) before starting WF and had resumed the diet on the day of WF initiation. After WF resumption, food intake was about 45% from day 1 to day 11, but it was about 66% from day 12 to day 18. Therefore, nutritional status was considered relatively good during WF treatment.
Prior to the WF restart, the patient was on antibiotic therapy with vancomycin and meropenem. Theoretically, antibiotics may increase PT-INR by altering the gastrointestinal flora, resulting in reduced vitamin K synthesis.25) However, whether containing an N-methylthiotetrazole (NMTT) side chain with inhibitory activity of the vitamin K epoxide reductase complex, subunit 1 (VKORC1) has been also reported to be more important in enhancing the anticoagulant effect of WF. 26,27) Vancomycin does not have NMTT side chain, and as far as we are aware, there are currently no reports of interactions between meropenem and WF, so it is unlikely that the patient's antimicrobial use affected the anticoagulant effect of WF.
Finally, the importance of two gene polymorphisms, CYP2C9 and VKORC1, for WF dosing are well established.28) The frequency of CYP2C9*3 allele, which reduces CYP2C9 metabolic activity in the Japanese population, is only 1.8%.29) Furthermore, 80% of East Asian patients have the VKORC1 genotype as WF low-dosage factor.28) Furthermore, the average WF dose in Japanese patients over 70 years of age has been reported as 2.6 ± 1.1 mg,30) but in the present patient, the dosage of WF was adjusted to 1.75 mg at outpatient visits before admission, and it was within the average range. Therefore, the influence of these genetic polymorphisms may be negligible.
In conclusion, we report a case of drug interaction induced by the combination of WF, amiodarone, and tramadol/acetaminophen. Even with continuing drug interaction, rapid PT-INR reduction was achieved by discontinuing WF and administering vitamin K1. Reasonable PT-INR control was achieved by appropriately reducing and restarting the WF dose. The intervention, in this case, may provide useful information for physicians and pharmacists involved in anticoagulation therapy. Further accumulation of multi-drug DDI cases that are difficult to obtain from large-scale clinical trials, such as this report, would be clinically beneficial.
Author contributionsMH, HS, JCST, and NK: wrote the manuscript. MH, EK, MI and YO: participated in the patient's evaluation and treatment. EK, MI, YO: review and editing the manuscript. All authors read and approved the final manuscript.
AcknowledgmentsWe would like to thank Editage (www.editage.com) for English language editing. This work was supported by JSPS KAKENHI Grant Number 22K06743.
Conflict of interestThe authors declare no conflict of interest.