Abstract
Parkinson's disease (PD) is a neurodegenerative disorder marked by progressive motor dysfunction. Dopaminergic neurons in the substantia nigra are likely the main cause of PD onset. The Hippo signaling pathway regulates organ size and tumor suppression. The Yes-associated protein (YAP) is a nuclear effector of the Hippo signaling pathway, and activation of YAP may be beneficial in several neurodegenerative diseases. In this study, we examined the effects of compound A [N-(tert-butyl)-2-(pyridin-4-yl)-1,7-naphthyridin-4-amine], a large tumor suppressor kinase (LATS) inhibitor, on 6-hydroxydopamine (6-OHDA)-induced cell damage in vitro and in vivo. In human neuroblastoma SH-SY5Y cells, compound A showed a protective effect against 6-OHDA-induced cell death without exhibiting any cytotoxicity. In order to investigate the effects of compound A on dopaminergic neurons, compound A was orally administrated to mice twice a day for 21 d. Next, mouse brains were harvested to assess the expression of (1) a dopaminergic neuron marker and (2) a YAP transcriptional target. Treatment of mice with 6-OHDA suppressed the expression of tyrosine hydroxylase (TH), a dopaminergic neuron marker, and compound A (3 mg/kg, per os) administration ameliorated the TH expression levels. In addition, compound A upregulated the mRNA expression levels of connective tissue growth factor (CTGF), a YAP transcriptional target. These results suggest that activation of the Hippo signaling pathway by LATS inhibition may be used as a novel therapeutic target for treating PD.
INTRODUCTION
Parkinson's disease (PD) is the second most common progressive neurodegenerative disease. PD appears to progress as a result of dopaminergic loss in the substantia nigra. PD is characterized by the following motor symptoms: tremors, bradykinesia, and rigid muscles. Already 60-70% of dopaminergic cells have been lost when motor symptoms appear in PD. Therefore, preventing dopaminergic neuron loss effectively suppresses the progression of PD symptoms.
The Hippo signaling pathway regulates organ size and was first discovered in Drosophila melanogaster. Similarly, in mammals, it appears to be associated with the modulation of organ size and tumor inhibition. These functions of the Hippo signaling pathway are mediated by its regulation of both apoptosis and cell proliferation.1,2) The Yes-associated protein (YAP) is the nuclear effector of this pathway, which activates a transcription factor referred to as the transcriptional enhancer activator domain (TEAD). The upregulation of YAP activity has also been linked to cell proliferation and cancer.3) On the other hand, a low incidence of cancer has been reported in several patients with the neurodegenerative disease.4,5) In addition, phosphorylation of the mammalian sterile 20 (STE20)-like kinase 1 (MST1), an upstream regulator of YAP, was increased in ventral motor neurons from amyotrophic lateral sclerosis patients.6) In Huntington’s disease, YAP is associated with transcriptional repression-induced atypical cell death (TRIAD).7,8) Collectively, these reports suggest that the Hippo signaling pathway is inactivated in several neurodegenerative diseases. Therefore, this pathway may be used as a novel target- for developing drugs to treat various neurodegenerative diseases.
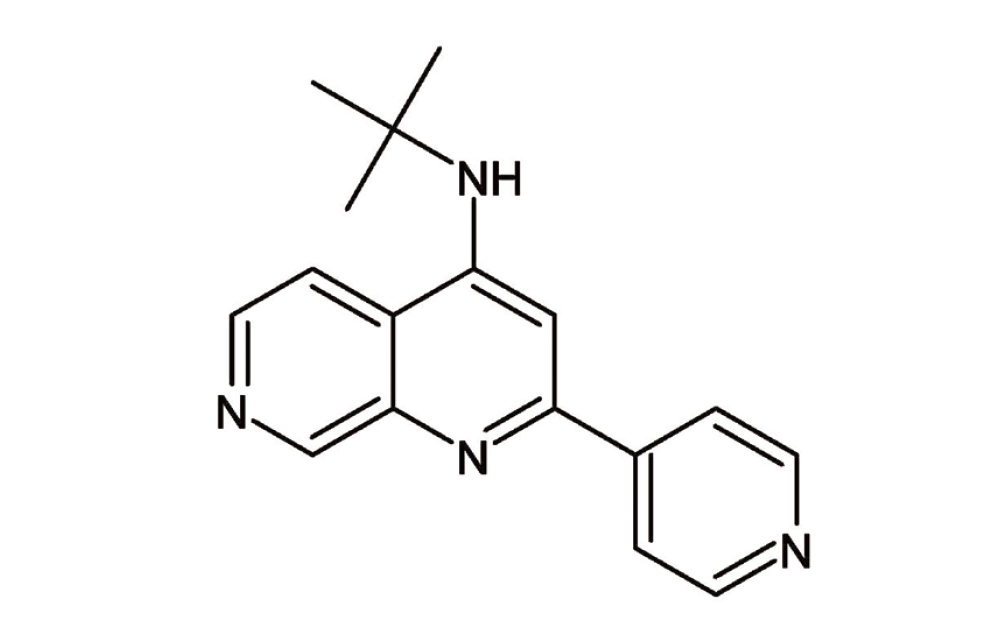
The large tumor suppressor kinases (LATS) 1 and LATS2 are regulators of the Hippo pathway. When the Hippo pathway is activated, the LATS1/2 kinases are phosphorylated and activated. Activated LATS1/2 directly phosphorylates and inactivates YAP, then phosphorylated YAP is subsequently degraded by the proteasome. LATS1/2 also inhibits the nuclear translocation of YAP/transcriptional co-activator with PDZ-binding motif (TAZ), which activates the Hippo pathway. Interestingly, in postmitotic mammalian tissues, the pharmacological inhibition of LATS promoted YAP-dependent proliferation.9) However, it is unclear whether pharmacological LATS inhibition has protective effects against neuronal cell death. In this study, we found that compound A [N-(tert-butyl)-2-(pyridin-4-yl)-1,7-naphthyridin-4-amine] (Fig. 1), which is a LATS inhibitor, protected against 6-hydroxydopamine (6-OHDA)-induced cell damage in a murine PD model. We then investigated the molecular mechanism responsible for compound A-mediated protection from cell damage in this PD model.
MATERIALS AND METHODS
Compounds
The LATS inhibitor compound A [N-(tert-butyl)-2-(pyridin-4-yl)-1,7-naphthyridin-4-amine] was synthesized10) by Nissan Chemical Corporation.
Kinase Assay
A kinase assay was conducted in 384-well black plates (Greiner Bio-One, Kremsmünster, Austria) using the Fluorospark® Kinase/ADP Multi-Assay kit (FUJIFILM Wako Pure Chemical Corporation, Kyoto, Japan) at a final volume of 5 µL/well according to the manufacturer’s protocol. A solution containing LATS1 (Carna Biosciences, Kobe, Japan) or LATS2 (Carna Biosciences) in assay buffer (20 mM HEPES, 5 mM MgCl2, 0.01%, 2 mM DTT, 50 µg/mL BSA, 0.01%Triton X-100, pH7.5) was dispensed into each well (5 ng/µL, 2 µL/well). Next, a solution (2 µL) containing compound A dissolved in assay buffer was added to each well (0.3-10000 nM). The reactions were initiated by adding 1 µL/well of a solution containing serum and glucocorticoid-regulated kinase-tide substrate (10 µM) and ATP (400 µM). The kinases were not added to the positive control wells, and the test compounds were not added to the negative control wells. The assay plates were incubated at room temperature for 5 h and the reactions were terminated. The samples were then, quantified using a Fluorospark® Kinase/ADP Multi-Assay kit, and an EnSpire (Perkin Elmer) was used to measure the fluorescence intensities at 540 nm (excitation) and 590 nm (emission). The fluorescence intensities of a test well (T), positive well (P), and negative well (N) were used to calculate the ratio of inhibition ([T-N]/[P-N]). IC50 value was calculated using EXSAS ver. 10.0 software (Arm Systex, Osaka, Japan).
Cell Culture
The human neuroblastoma SH-SY5Y cell line was obtained from the European Collection of Cell Culture (Wiltshire, UK). The cells were cultured in Dulbecco’s modified Eagle’s medium (D-MEM; Nacalai tesque, Kyoto, Japan) containing 10% fetal bovine serum (FBS), 100 μg/mL penicillin and 100 units/mL streptomycin (Meiji Seika Co. Ltd., Tokyo, Japan). The culture environment was maintained at 37°C, with 95% air and 5% CO2 in a humid atmosphere. The cells were passaged by trypsin treatment to 80% confluency and were replaced with fresh medium every 2 or 3 d.
Cell Viability Assay
SH-SY5Y cells were seeded in a 96-well plate at 1 × 104 cells per well. After incubation for 24 h, the entire medium was replaced with fresh medium containing 1% FBS. Compound A was then added to the culture medium and incubated for 24 h. The cell viability was measured using a CCK-8 assay following the manufacturer’s instructions (Dojindo, Kumamoto, Japan). After treatment with compound A, 10 μL of CCK-8 solution was added to each well. The plate was incubated at 37°C for 2 h, and the absorbance was measured at 450 nm using a Varioskan Flash 2.4 microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) at a reference wavelength of 630 nm.
Cell Death Assay
SH-SY5Y cells were seeded in a 96-well plate at 1 × 104 cells per well. After incubation for 24 h, the entire medium was replaced with fresh medium containing 1% FBS. Compound A or N-acetyl cysteine (NAC), used as positive control, was added to the culture medium and the cells were incubated for 1 h. 6-OHDA (Sigma-Aldrich, St. Louis, MO, USA) was then added to the culture medium followed by a 12 h incubation. The cells were evaluated with a nuclear staining assay. Cell death was assessed by combined staining with Hoechst 33342 (Molecular Probes, Eugene, OR, USA) and propidium iodide (PI; Molecular Probes). The cell images were collected using a Lionheart microscope (BioTek Instruments, Inc, Winooski, VT, USA). The cell counts per condition were quantitated with Gen5 software (version 3.03).
Animals
In this study, we utilized eight-week-old male C57BL/6J mice (Japan SLC, Hamamatsu, Japan). The mice were kept under a 12 h/12 h light/dark cycle (lights on from 8:00 to 20:00) at 24 ± 2ºC. The mice had free access to food and water. All procedures for animal care and treatment were conducted following the animal care guidelines published by the Gifu Pharmaceutical University Animal Experiment Committee.
6-OHDA Lesioned Model
Hemi injection of 6-OHDA was performed according to a previous report.11) Briefly, mice were anesthetized with ketamine (43.8 mg/kg) and xylazine (2.5 mg/kg) by intraperitoneal administration. Under anesthesia, the skull of the mouse was exposed, and the drug was injected (2 µL) at the following points: anterior-posterior (AP +0.5 mm), lateral (L -2.0 mm, left), and dorsal-ventral (DV -3.0 mm) from the bregma. The 6-OHDA solution (5 µg/µL in 0.9% NaCl with 0.02% ascorbic acid) was injected over 3 min. Control animals were administered 0.9% NaCl and 0.02% ascorbic acid. Compound A was suspended in 0.5% methylcellulose and administered orally (10 mL/kg) twice a day for 3 weeks. After 3 weeks of compound A administration, the mice were subsequently sacrificed and the cerebral cortex and striatum were harvested.
Western Blot Analyses
Mouse brains were lysed in radioimmunoprecipitation buffer (RIPA buffer) [150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1% Igepal CA-630, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS)] containing protease (Sigma Aldrich) and phosphatase inhibitor cocktails 1 and 2 (Sigma Aldrich). The lysates were centrifuged at 12,000 g for 20 min at 4°C. The protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) with a bovine serum albumin standard. SDS sample buffer (Fujifilm Wako Chemicals) was added to the lysates followed by a reaction at 90°C for 5 min. Subsequently, the samples were subjected to 5-20% SDS-polyacrylamide gel electrophoresis, and the electrophoresed proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, MA, USA). The membrane was incubated with the following primary antibodies: tyrosine hydroxylase (TH) mouse monoclonal antibody (Santa Cruz; 1:1000 dilution) and β-actin mouse monoclonal antibody (Sigma Aldrich; 1:5000 dilution). Next, the membrane was incubated with horse radish peroxidase-conjugated goat anti-mouse IgG secondary antibody (Pierce Biotechnology; 1:1000 dilution). The immunoreactive bands were detected with an Amersham Imager 680 (Cytiva, Tokyo, Japan.).
mRNA Expression Analyses
Total RNA was isolated from mouse brains using the ISOGEN II reagent (#311-07361, NIPPONGENE) and the RNeasy Mini kit (#74106, QIAGEN) according to the manufacturer’s protocol. Briefly, total RNA was extracted from whole brain homogenates with ISOGEN II followed by RNA purification using RNeasy columns. Approximately 20 ng of total RNA was used to produce cDNA with the PrimeScript RT reagent kit (#RR037A, TaKaRa) following the manufacturer’s protocol. Reverse transcription-quantitative polymerase Chain Reaction (RT-qPCR) was then performed using the Premix Ex Taq kit (#RR039A, TaKaRa) on an Applied Biosystems 7500 Real-Time PCR System. The cDNA sample was diluted 5-fold with nuclease-free water. The PCRs were performed at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 34 s. The data were analyzed by the delta-delta C(t) method as previously described.12) The RT-qPCR TaqMan probes consisted of connective tissue growth factor (CTGF; Mm01192933_g1) and Gapdh (Mm99999915_g1).
Statistical Analyses
The data are expressed as the mean ± standard error of the mean (SEM). The statistical analyses were conducted using the Student’s t-test or one-way ANOVA followed by Dunnett's test. P < 0.05 were assumed to be statistically significant. Statistical Package for Social Science (SPSS) Statistics software (IBM, Armonk, NY, USA) was used to perform the statistical analyses.
RESULTS
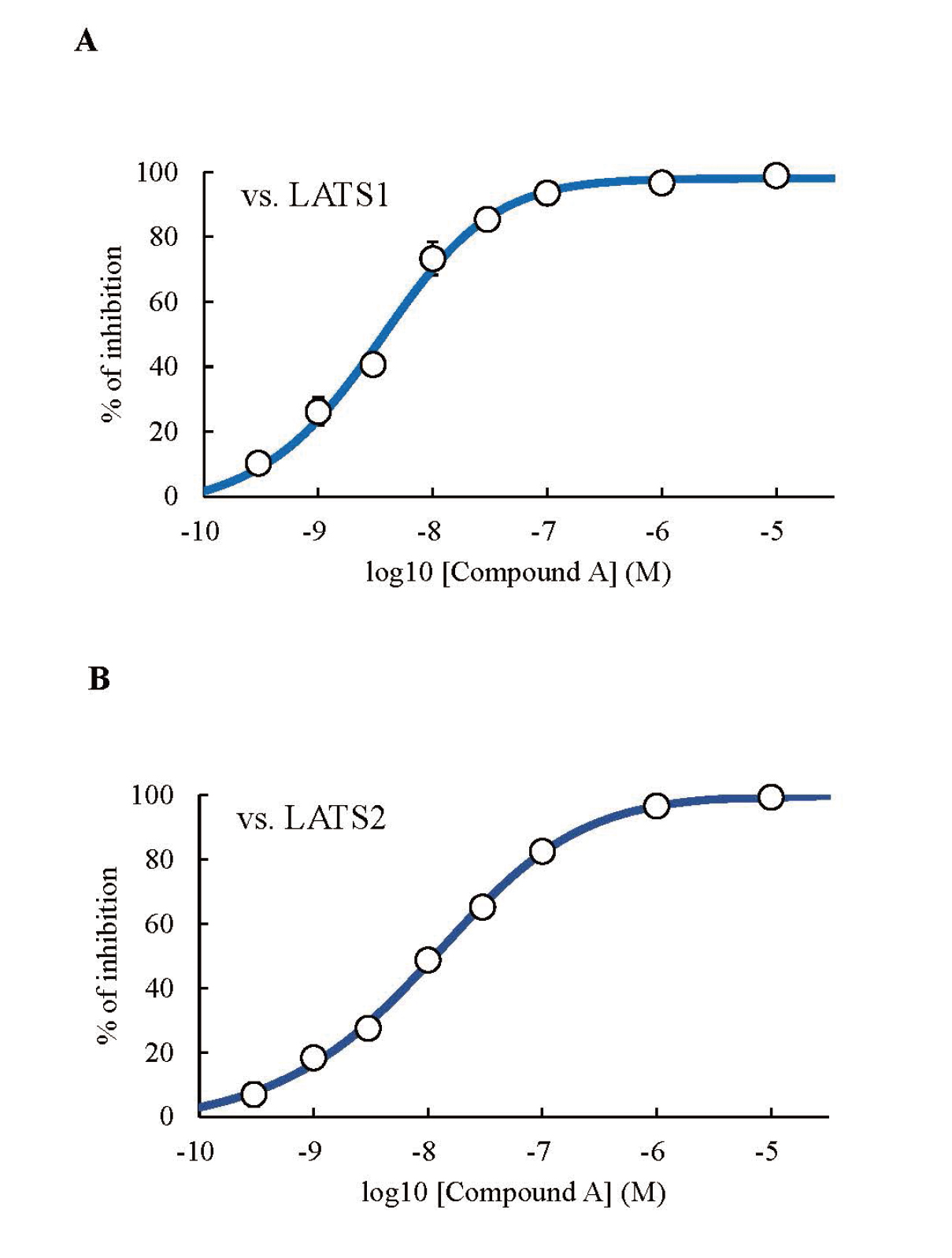
The LATS1 and LATS2 Inhibitory Activities of Compound A
Firstly, we calculated the LATS1 and LATS2 inhibitory activities of compound A using a kinase assay. The IC50 values of compound A against LATS1 and LATS2 were 3.95 ± 0.70 and 11.39 ± 1.69 nM, respectively (Figs. 2A, B).
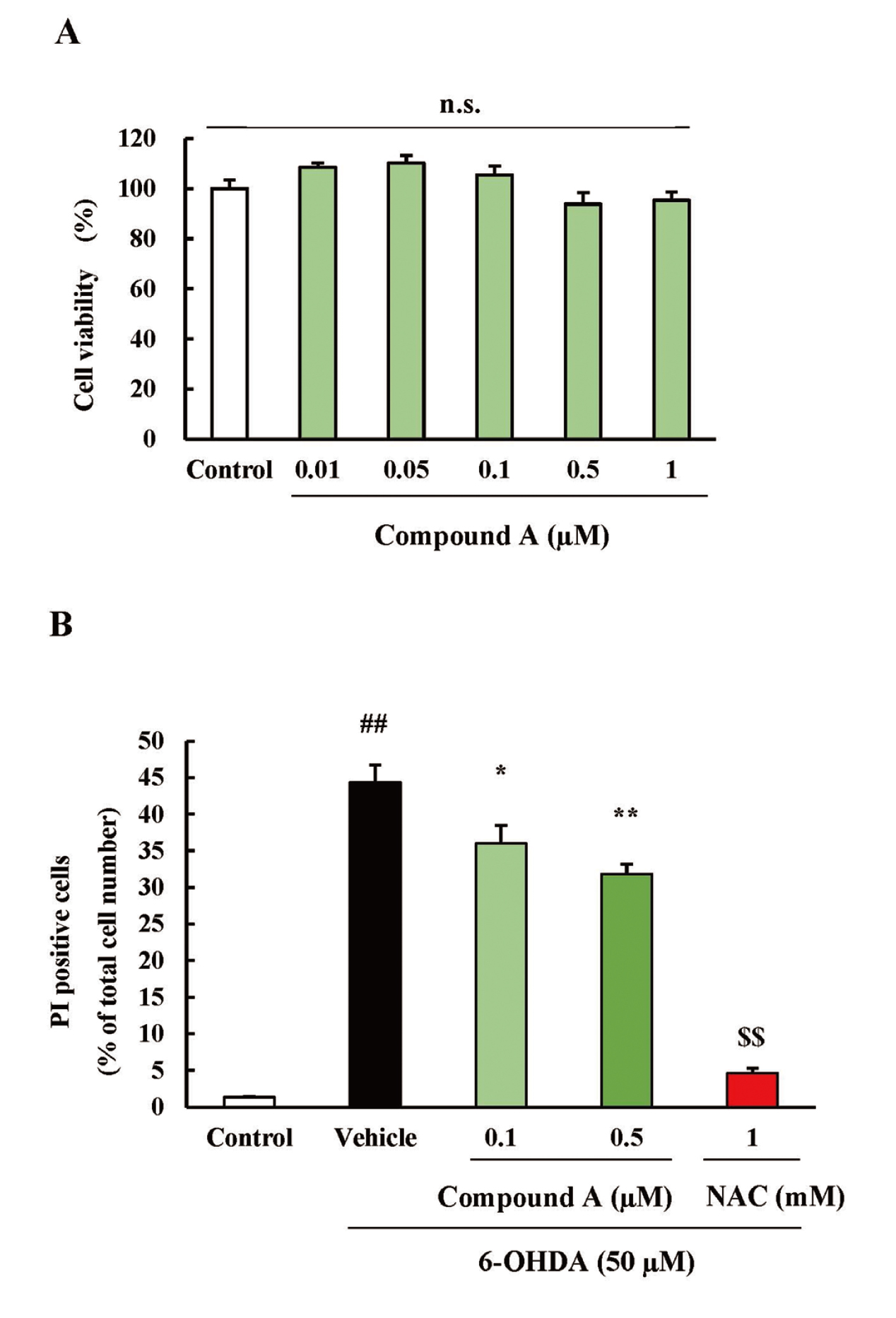
The Effects of Compound A on SH-SY5Y Cell Viability
Before examining the effects of compound A on 6-OHDA-induced SH-SY5Y cell death, the effects of compound A on cell viability activity were tested. Treatment with compound A (0.01-1 µM) alone for 24h did not decrease cell viability. This result suggested that compound A (0.01-1 µM) is not cytotoxic towards SH-SY5Y cells (Fig. 3A).
The Effect of Compound A on 6-OHDA-Induced SH-SY5Y Cell Death
We examined the effects of compound A on 6-OHDA-induced SH-SY5Ycell death. Treatment of SH-SY5Y with 50 µM of 6-OHDA significantly increased the cell death rate. Compound A (0.1 and 0.5 µM) significantly inhibited SH-SY5Y cell death induced by 6-OHDA in a concentration-dependent manner. N-acetylcysteine (NAC), used as a positive control, also significantly inhibited SH-SY5Y cell death induced by 6-OHDA (Fig. 3B).
The Effects of Compound A on the Expression of a Dopaminergic Neuron Marker
We examined the effects of compound A on a dopaminergic neuron from the murine striatum. Injection of mice with 6-OHDA selectively decreased the expression level of the dopaminergic neuron marker, TH (Figs. 4A, B). Interestingly, compound A (3 mg/kg) significantly ameliorated the TH expression level in the side of the brain injected with 6-OHDA (Figs. 4C, D).
The Effects of Compound A on the Transcriptional Target of YAP
We hypothesized that compound A enhanced the expression level of TH by promoting YAP-mediated transcription. Therefore, we injected mice with 6-OHDA and monitored the mRNA expression of CTGF, a transcriptional target of YAP. Injection of mice with 6-OHDA injection had no effect on the CTGF mRNA levels. Interestingly, compound A (3 mg/kg) significantly increased the CTGF mRNA levels compared to that observed in 6-OHDA-treated mice (Fig. 5).
DISCUSSION
In this study, we examined the effects of compound A on 6-OHDA-induced cell damage in vitro and in vivo. Firstly, we evaluated the effects of compound A alone on SH-SY5Y cell viability. Our CCK-8 assay results showed that treatment of SH-SY5Y with compound A (0.01-1 µM) for 24 h did not affect cell viability. This result suggested that compound A has no proliferative activity and no cytotoxicity against SH-SY5Y.
6-OHDA is widely used to mimic a model of PD model in vitro and to evaluate the protective effects of candidate drugs for treating PD.13-15) In SH-SY5Y cells, 6-OHDA induces apoptosis via activation of the PI3K-Akt signaling pathway.16) The Hippo pathway is one of the mechanisms that regulate apoptosis not only in tumors but also in several neurodegenerative diseases tissues.17) YAP exhibits anti-apoptotic effects through the expression of B-cell lymphoma-extra large (Bcl-xL), an anti-apoptotic protein that prevents the release of mitochondrial contents such as cytochrome c from cancer cells.18) Therefore, one possible mechanism for compound A, increased Bcl-xL expression may have protected against 6-OHDA-induced apoptosis. However, the mechanism of action of compound A needs to be further elucidated in future studies.
6-OHDA is also used to induce an in vivo PD model in mice and rats. Since 6-OHDA cannot cross the blood-brain barrier, cell damage is induced by the injection of 6-OHDA directly into the brain. 6-OHDA enters dopaminergic neurons via the dopamine transporter and selectively damages the dopaminergic neurons by ROS production. In this study, we injected 6-OHDA into the left side of the striatum (ipsilateral). We observed that the 6-OHDA injection induced a site-specific decrease in the expression level of TH, a dopaminergic neuron marker. This result suggested that the dopaminergic neuron population was selectively decreased on the side of the brain injected with 6-OHDA. Compound A (3 mg/kg, per os; p.o.) ameliorated the TH expression levels in 6-OHDA-injected mice brains. It has been reported that YAP activation promotes dopaminergic neuron survival and differentiation.19) In addition, MST1/2, upstream of LATS, phosphorylation promotes dopaminergic neuron death.20) These reports and our findings suggest that compound A reduced 6-OHDA-induced dopaminergic neuron damage by (1) reducing oxidative stress and (2) promoting dopaminergic neuron survival by inhibiting LATS.
There is a difference between in vitro and in vivo effects. In vitro studies have shown little difference in the protective effect of compound A at 0.5 µM and 1 µM (data not shown), suggesting that the direct effect of compound A on neurons is almost at its peak at 0.5 µM. On the other hand, the strong in vivo effect at 3 mg/kg may be due to the fact that compound A acts on cells other than neurons and may have an additive inhibitory effect on cell death. Astrocytes express a cysteine/glutathione exchange transporter that provides glutathione to neurons. 6-OHDA is taken up by neurons, induces oxidative stress, and depletes glutathione. In addition, 6-OHDA administration causes astrogliosis around the site of administration. Levetiracetam and zonisamide have been reported to exhibit dopaminergic neuroprotective effects in 6-OHDA-induced rodent PD models by promoting astrocyte proliferation and increasing glutathione synthesis.21,22) LATS1 is also involved in astrocyte proliferation, and knockdown of LATS1 in primary astrocyte culture cells by siRNA promotes cell growth.23) Thus, compound A may have promoted astrocyte proliferation by inhibiting LATS1 and suppressed neuronal cell death via glutathione supply. However, the action of LATS inhibition in astrocytes is unknown and should be investigated in detail in the future.
YAP translocate from the cytoplasm to the nucleus and induces the transcription of various genes. CTGF is one of the transcriptional targets of YAP. Overexpression of CTGF expression contributes to the resistance of cancer cells to anti-cancer drug-induced cell death.24) The LATS inhibitor, TLURI, upregulates Ctgf gene expression in utricular supporting cells.9) This report is consistent with the present results, in which administration of compound A (3 mg/kg, p.o.) to PD model mice increased the expression of CTGF mRNA (Fig. 5). The CTGF protein acts as a neurotrophic factor via activation of the p44/42 mitogen-activated protein kinase (MAPK) signaling pathway.25) Since p44/42 mediates cell proliferation and neurogenesis of neural progenitor cells,26) increased expression of CTGF may also protect dopaminergic neurons through the promotion of cell proliferation and neurogenesis. These results may indicate that YAP activation by LATS inhibition activates p44/42 via transcriptional upregulation of CTGF, which reduced 6-OHDA-induced dopaminergic nerve damage. However, further studies are required to elucidate the detailed mechanism of compound A-mediated cell protection.
In conclusion, compound A was effective against 6-OHDA-induced dopaminergic cell damage in vitro and in vivo. These findings suggest that compound A may be a new therapeutic agent targeting the Hippo signaling pathway in PD.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1) Pan D. The hippo signaling pathway in development and cancer. Dev. Cell, 19, 491–505 (2010).
- 2) Santucci M, Vignudelli T, Ferrari S, Mor M, Scalvini L, Bolognesi ML, et al. The hippo pathway and YAP/TAZ-TEAD protein-protein interaction as targets for regenerative medicine and cancer treatment. J. Med. Chem., 58, 4857–4873 (2015).
- 3) LaQuaglia MJ, Grijalva JL, Mueller KA, Perez-Atayde AR, Kim HB, Sadri-Vakili G, et al. YAP subcellular localization and hippo pathway transcriptome analysis in pediatric hepatocellular carcinoma. Sci. Rep., 6, 30238 (2016).
- 4) Gibson SB, Abbott D, Farnham JM, Thai KK, McLean H, Figueroa KP, et al. Population-based risks for cancer in patients with ALS. Neurology, 87, 289–294 (2016).
- 5) Katisko K, Haapasalo A, Koivisto A, Krüger J, Hartikainen P, Korhonen V, et al. Low prevalence of cancer in patients with frontotemporal lobar degeneration. J. Alzheimers Dis., 62, 789–794 (2018).
- 6) Lee JK, Shin JH, Hwang SG, Gwag BJ, McKee AC, Lee J, et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc. Natl. Acad. Sci. USA, 110, 12066–12071 (2013).
- 7) Hoshino M, Qi ML, Yoshimura N, Miyashita T, Tagawa K, Wada Y, et al. Transcriptional repression induces a slowly progressive atypical neuronal death associated with changes of YAP isoforms and p73. J. Cell Biol., 172, 589–604 (2006).
- 8) Mao Y, Chen X, Xu M, Fujita K, Motoki K, Sasabe T, et al. Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology. Hum. Mol. Genet., 25, 4749–4770 (2016).
- 9) Kastan N, Gnedeva K, Alisch T, Petelski AA, Huggins DJ, Chiaravalli J, et al. Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues. Nat. Commun., 12, 3100 (2021).
- 10) Behnke D, Berenshteyn F, Hao X, Hoffman TZ., Jin Q, Lacoste A, Lee C, Liu J, Liu YA, Maibaum JK, Mo T, Pan J, Qu X, Tchorz J, Xie YF, Yan S, Zou Y. 6-6 Fused bicyclic heteroaryl compounds and their use as lats inhibitors, (2018).
- 11) da Conceição FS, Ngo-Abdalla S, Houzel JC, Rehen SK. Murine model for Parkinson’s disease: from 6-OH dopamine lesion to behavioral test. J. Vis. Exp. (2010).
- 12) Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25, 402–408 (2001).
- 13) Giuliani P, Romano S, Ballerini P, Ciccarelli R, Petragnani N, Cicchitti S, et al. Protective activity of guanosine in an in vitro model of Parkinson’s disease. Panminerva Med., 54, 43–51 (2012).
- 14) Xicoy H, Brouwers JF, Kalnytska O, Wieringa B, Martens GJM. Lipid analysis of the 6-hydroxydopamine-treated SH-SY5Y cell model for Parkinson’s disease. Mol. Neurobiol., 57, 848–859 (2020).
- 15) Ferlazzo N, Cirmi S, Maugeri A, Russo C, Lombardo GE, Gangemi S, et al. Neuroprotective effect of bergamot juice in 6-OHDA-induced SH-SY5Y cell death, an in vitro model of Parkinson’s disease. Pharmaceutics, 12, (2020).
- 16) Watanabe R, Kurose T, Morishige Y, Fujimori K. Protective effects of fisetin against 6-OHDA-induced apoptosis by activation of PI3K-akt signaling in human neuroblastoma SH-SY5Y cells. Neurochem. Res., 43, 488–499 (2018).
- 17) Sahu MR, Mondal AC. The emerging role of Hippo signaling in neurodegeneration. J. Neurosci. Res., 98, 796–814 (2020).
- 18) Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet., 47, 250–256 (2015).
- 19) Zhang D, Yang S, Toledo EM, Gyllborg D, Saltó C, Carlos Villaescusa J, et al. Niche-derived laminin-511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci. Signal., 10, (2017).
- 20) Ahn EH, Kang SS, Qi Q, Liu X, Ye K. Netrin1 deficiency activates MST1 via UNC5B receptor, promoting dopaminergic apoptosis in Parkinson’s disease. Proc. Natl. Acad. Sci. USA, 117, 24503–24513 (2020).
- 21) Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, et al. Neuroprotective effects of zonisamide target astrocyte. Ann. Neurol., 67, 239–249 (2010).
- 22) Miyazaki I, Murakami S, Torigoe N, Kitamura Y, Asanuma M. Neuroprotective effects of levetiracetam target xCT in astrocytes in parkinsonian mice. J. Neurochem., 136, 194–204 (2016).
- 23) Wang Y, Chen M. Decreased expression of LATS1 correlates with astrogliosis after spinal cord injury. Biochem. Biophys. Res. Commun., 505, 151–156 (2018).
- 24) Yang K, Gao K, Hu G, Wen Y, Lin C, Li X. CTGF enhances resistance to 5-FU-mediating cell apoptosis through FAK/MEK/ERK signal pathway in colorectal cancer. OncoTargets Ther., 9, 7285–7295 (2016).
- 25) Shoji M, Ueda M, Nishioka M, Minato H, Seki M, Harada K, et al. Jiadifenolide induces the expression of cellular communication network factor (CCN) genes, and CCN2 exhibits neurotrophic activity in neuronal precursor cells derived from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun., 519, 309–315 (2019).
- 26) Liu HY, Chen CY, Hung YF, Lin HR, Chao HW, Shih PY, et al. RNase A promotes proliferation of neuronal progenitor cells via an ERK-dependent pathway. Front. Mol. Neurosci., 11, 428 (2018).