2023 Volume 6 Issue 4 Pages 150-154
2023 Volume 6 Issue 4 Pages 150-154
Enterobacter cloacae are thought to exhibit resistance to third-generation cephalosporins (3GCs) despite their sensitivity to these drugs; therefore, broad-spectrum antibiotics such as carbapenems are primarily used against them. Reports elucidating the treatment outcomes of 3GCs against E. cloacae are limited; therefore, we aimed to accumulate further evidence in this regard. Patients with isolated E. cloacae infections who were treated with antibiotics between April 2008 and April 2021 at Hokkaido University Hospital were included in the study. The primary endpoint was the difference in treatment efficacy between patients treated with the 3GCs after the detection of E. cloacae and those treated with other drugs. As a secondary endpoint, we compared the differences in treatment efficacy rates according to specimen type, severity, and type of antibiotics. Furthermore, multivariate analysis was performed to identify the factors influencing treatment failure. Among the 146 cases analyzed, 25 and 121 were categorized into the 3GCs and others groups, respectively. The treatment efficacy rate did not significantly differ between the two groups (3GC vs. others: 80.0% vs. 84.3%; P = 0.564). Only one patient in the 3GCs group developed resistance. The treatment efficacy rate did not differ according to the specimen type, severity, or antibiotic class between the groups. Multivariate analysis confirmed that the use of 3GCs did not affect treatment failure. 3GCs may be considered a potential therapeutic option for E. cloacae infections.
Enterobacter cloacae is a resident intestinal bacterium that can cause healthcare-associated infections in immunocompromised individuals. E. cloacae harbor a chromosomal AmpC β-lactamase gene that is normally suppressed but can be induced to express and develop resistance to β-lactams, especially third-generation cephalosporins (3GCs).1) Other bacteria that have chromosomal AmpC β-lactamase genes include Enterobacter spp., Citrobacter freundii, Serratia marcescens, and Morganella morganii, but Enterobacter spp. reportedly has the highest frequency of resistance development.2) Carbapenems are the first-line treatment for Enterobacter spp.; however, the rate of carbapenem resistance is increasing.3) Cefepime is a weak and stable inducer of AmpC,4) and its mortality rate is not inferior to that of carbapenems; therefore, it is used as an alternative drug.5) 3GCs are also weak inducers of AmpC, but their use is not recommended because they are labile to AmpC, can cause resistance, and can influence treatment failure.4,6,7,8) However, the frequency of 3GC usage-related development of resistance is relatively low and unrelated to treatment failure.9,10) Studies that compared the treatment results of 3GCs and other antibiotics revealed no differences in mortality rates.11) However, many of these studies included S. marcescens and M. morganii, which are at low risk of AmpC overproduction. Therefore, the results should be interpreted with caution. Furthermore, reports on the comparison of treatment outcomes of 3GCs and other antibiotics in E. cloacae, a high-risk AmpC-producing bacterium, are limited.
Therefore, we aimed to accumulate evidence to support the use of 3GCs in E. cloacae.
We conducted a retrospective study on individuals diagnosed with E. cloacae infection and treated with antibiotics from April 2008 to April 2021 at Hokkaido University Hospital, Japan. Inclusion criteria were patients aged 18 years or older and those with E. cloacae susceptible to 3GCs. The exclusion criteria were as follows: (1) simultaneous infection with bacteria other than E. cloacae; (2) no sensitive antibiotics used within 72 h of culture; and (3) use of multiple sensitive antibiotics for over 72 h. This research was conducted with permission from the Ethics Review Committee of Hokkaido University Hospital (study protocol NO. 021-0140).
Study EndpointsThe primary endpoint was the difference in treatment efficacy between patients treated with 3GCs after the detection of E. cloacae and those treated with other drugs. The secondary endpoints were the differences in therapeutic efficacy rates according to specimen type, severity, and type of antibiotics.
Evaluation of Efficacy and SeverityWe evaluated efficacy using the Guidelines on Clinical Evaluation Methods for Antimicrobials.12) Severity was assessed using quick sequential organ failure assessment (SOFA) scores for bacteremia and A-DROP or I-ROAD scores for pneumonia.13-15) Patients requiring surgical treatment for urinary tract infections were classified as severe.
Statistical AnalysisStatistical analysis was performed using the Mann–Whitney U test for continuous variables and Pearson’s chi-square or Fisher’s exact test for categorical variables. P values ≤ 0.05 were considered statistically significant.
The Bonferroni method was used to correct the P values for multiple comparisons to compare the treatment efficacy rate by antibiotic class. Factor analysis was performed using the presence or absence of treatment failure as the dependent variable and the factors that may influence treatment failure as the independent variables. Univariate analysis was performed for all risk factors associated with treatment failure, and factors with P ≤ 0.1 were included in the multivariate analysis. All statistical analyses were performed using EZR software.16)
A total of 146 patients were included in the study out of 450 patients (Fig. 1). Of the 146 patients, 25 were treated with 3GCs, and 121 were treated with other antibiotics. Their characteristics are listed in Table 1. Immunosuppression status, abscess formation, and absence of drainage, positive in blood culture or biliary tract infection were significantly different between the groups.
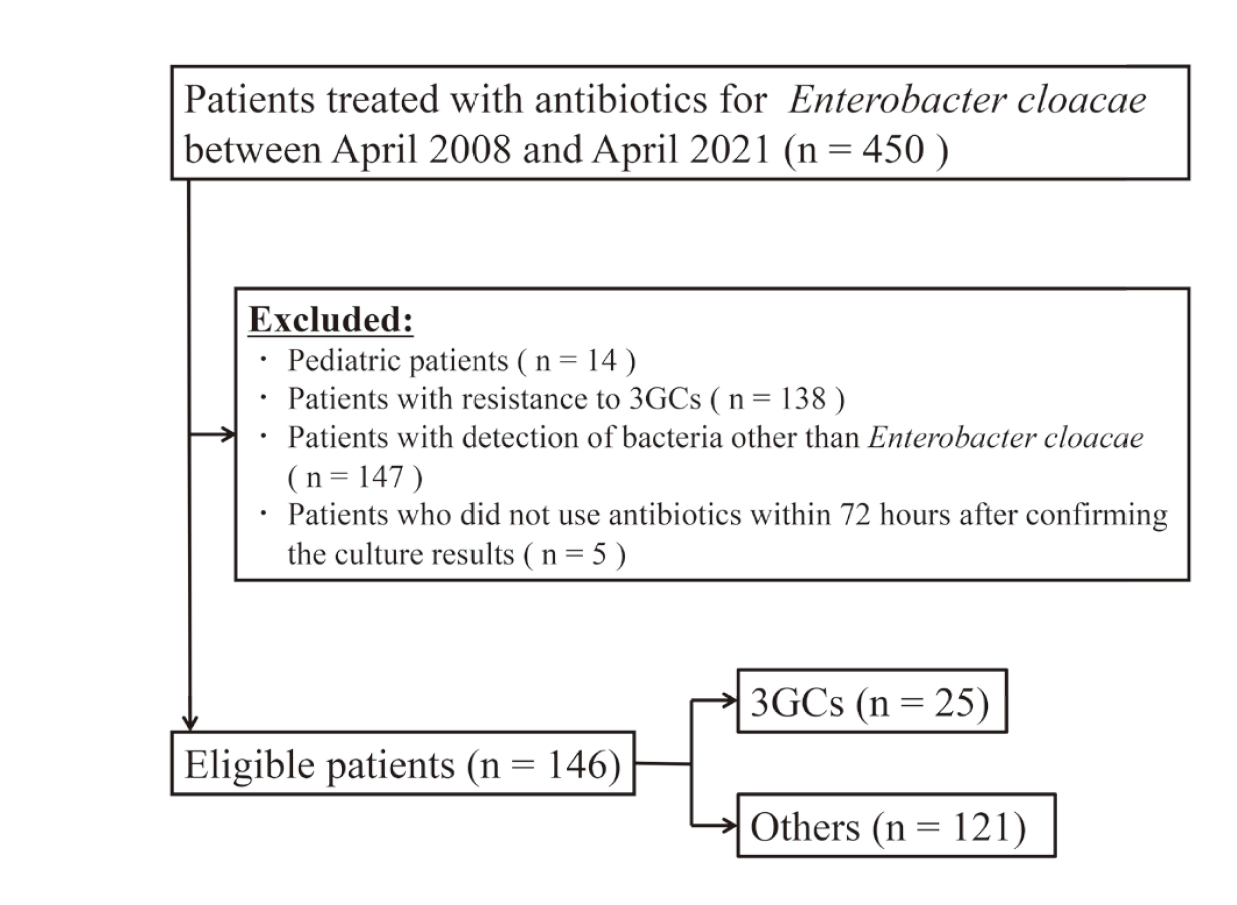
Flowchart of Patients Included in This Study
3GCs: third-generation cephalosporins.
 Treatment Efficacy
Treatment Efficacy
Efficacy rates in the 3GCs and others groups are presented in Table 2, and the breakdown of the antibiotics is listed in Table 3. The treatment efficacy rate did not significantly differ between the 3GCs and others groups (80.0% vs. 84.3%; P = 0.564). Only one patient in the 3GCs group developed resistance. The treatment efficacy rate did not significantly differ according to specimen type or disease severity between the groups (Table 2). Furthermore, the efficacy rate did not differ between each class of antibiotics (Fig. 2).

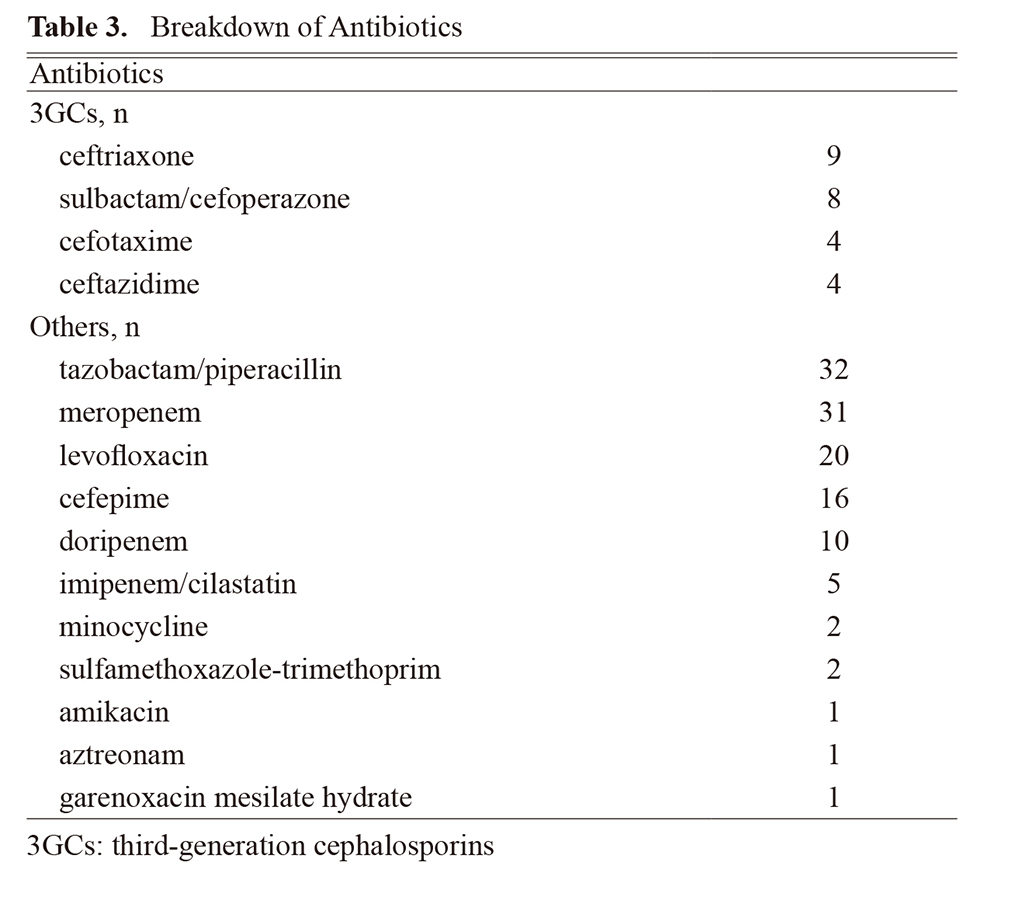

Efficacy Rate by Class of Antibiotics
3GCs: third-generation cephalosporins, 4GCs: fourth-generation cephalosporins, TAZ/PIPC: tazobactam/piperacillin. Fisher’s exact test, †P values were adjusted by Bonferroni correction.
Table 4 presents the results of the univariate analysis. Critically ill patients and antibiotics used within 90 days prior to initiating 3GCs or non-3GCs were included in the multiple logistic regression analysis (P ≤ 0.1; Table 5). Antibiotics used within 90 days before initiating 3GCs or non-3GCs were extracted as independent factors for treatment failure.
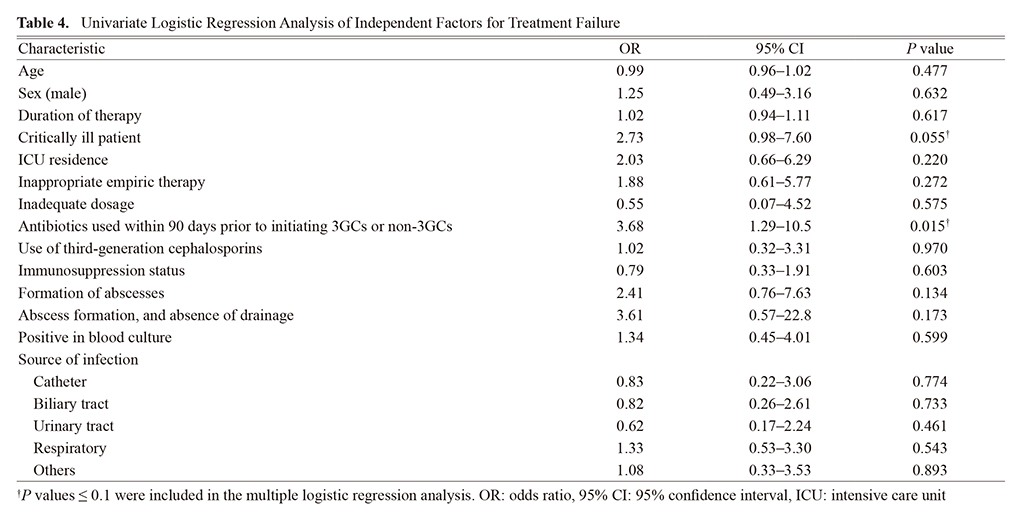
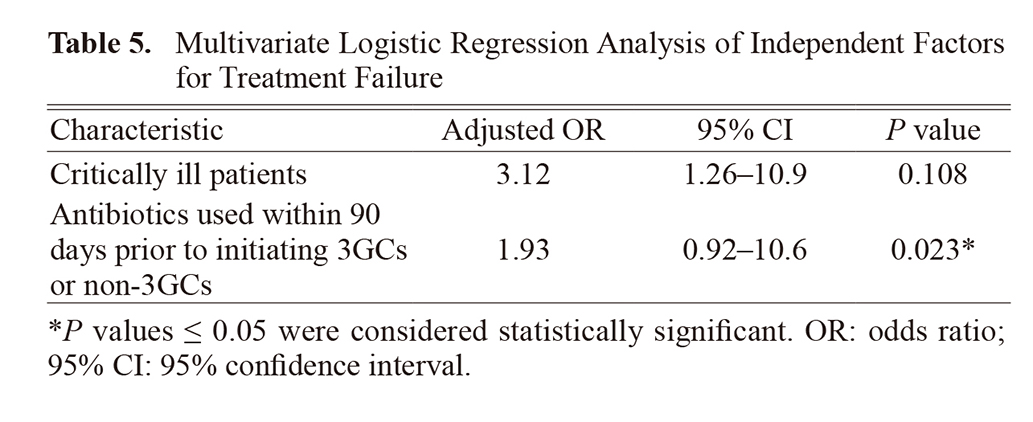
In this study, we investigated the efficacy of 3GCs against E. cloacae, as this antibiotic class is typically not recommended owing to concerns regarding resistance;8) however, some recent reports suggest that they do not affect treatment failure.9,10) Therefore, we compared the therapeutic efficacy rate of 3GCs and other antibiotics against E. cloacae and observed that patient characteristics, such as immunosuppression status, abscess formation and no drainage, positive in blood culture, and biliary tract infection were significantly different between the groups. However, the severity of infection did not differ between the two groups, and these items were not extracted as risk factors for treatment failure. Therefore, it is unlikely that they affected the results.
The therapeutic efficacy rate did not significantly differ between the patients treated with 3GCs and those treated with other drugs, and the results according to specimen type, severity, and antibiotic class were also similar.
Broad-spectrum antibiotics, such as carbapenems, are often used in patients with severe illness. Despite initial concerns that these antibiotics might affect the results of our study, we observed that there was no significant difference in severity between the two groups, suggesting that 3GCs may be used regardless of severity. In contrast, the Infectious Diseases Society of America (IDSA) guidelines state that 3GCs should not be used in cases of invasive infections.8) However, in uncomplicated cases such as no abscess formation where long-term use is not anticipated, the use of 3GCs may be considered even in severe cases.
We observed that 4GCs and carbapenems, as well as quinolones, were not significantly different from 3GCs in terms of efficacy rate (Fig. 2). Fluoroquinolones are a therapeutic option because they are not substrates for AmpC.8) However, we believe that caution should be exercised in their administration owing to their broader spectrum compared with that of 3GCs. Similar results were obtained for tazobactam/piperacillin; however, its use for severe infections is not recommended because of limited efficacy.8) Additionally, its spectrum is broader than that of 3GCs; therefore, its use is minimal clinical significance.
Multivariate analysis revealed that antibiotics used within 90 days prior to initiating 3GCs or non-3GCs was a risk factor for treatment failure, but the use of 3GCs was not. This suggests that 3GCs are unlikely to result in treatment failure and may be considered a therapeutic option for treating E. cloacae infections. Additionally, although we excluded cases of polymicrobial infections to rule out the effects of other bacteria, similar results may be obtained for infections in which E. cloacae is the primary causative organism.
Only one patient in the 3GCs group developed resistance, and the rate of resistance development did not differ between the two groups (3GCs group: 4.0% vs. others group: 0.0%, P = 0.171; data not shown). In other reports, the emergence rate of resistance was approximately 10%.17,18) This was higher than that observed in our study, which may be attributed to the longer duration of treatment in the other reports. However, we could not draw comparisons because the number of patients that developed resistance in our study was small. This resistant case was that of a liver abscess in a patient with cholangiocarcinoma. Previous reports have suggested that biliary tract infections associated with cholangiocarcinoma are one of the risk factors for the development of drug resistance.2) The results of this study are, therefore, reasonable. 3GCs may be withheld in cases of abscess formation. Additionally, the insufficient dosage is associated with AmpC overproduction.19) In this case, the antibiotic used was ceftazidime (CAZ) at a dose of 1 g every 8 h. CAZ is a renal excretion-type drug, and in this case, we observed no compromise in renal function. Therefore, the dosage could be increased to 2 g every 8 h. As shown in Table 3, several of the 3GCs used were ceftriaxone and sulbactam/cefoperazone, which are hepatically metabolized drugs. This particular class of drug is less likely to be underdosed compared with renal excretion-type drugs, which could potentially explain the lack of differences in the treatment efficacy rate. When using 3GCs against E. cloacae, the maximum dose of antibiotics should be administered, and hepatically metabolized drugs may be appropriate for patients with accelerated renal clearance. Thus, we believe that 3GCs can be used in selected cases.
This study had some limitations. The duration of treatment in the 3GCs group was 3–14 days, and cases with longer durations could not be evaluated. The longer the duration of therapy, the more likely it is that resistance will develop, leading to treatment failure. Additionally, securing an adequate number of cases required for a non-inferiority trial becomes imperative in assessing the presence of significant differences in the therapeutic efficacy rate. However, we could not collect the required number of cases required to conduct a non-inferiority trial; therefore, a multi-center collaborative study must be conducted in the future to overcome this limitation. Moreover, as this was an observational study and AmpC production could not be measured, we could not assess whether the overproduction of AmpC was directly related to treatment failure. Finally, the results of this study did not provide sufficient evidence to definitively recommend the use of 3GCs for the treatment of E. cloacae infection. Nonetheless, we believe that 3GCs can be an option in cases where the treatment duration is not prolonged and sufficient antibiotic concentrations can be achieved.
Our findings confirm the efficacy of 3GCs against E. cloacae infection. In appropriate cases with shorter treatment duration, 3GCs may be considered a therapeutic option, contributing to the reduced utilization of broad-spectrum antibiotics, such as carbapenems.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare no conflict of interest.