2023 Volume 6 Issue 6 Pages 176-182
2023 Volume 6 Issue 6 Pages 176-182
Background: Vitamin K derivatives have an important role in bone formation and blood clotting. Vitamin K2 [menaquinone-4 (MK-4)] is used for the treatment and prevention of osteoporosis. Recently, vitamin K was found to be effective for the prevention of osteoarthritis and may play a role in cartilage formation; however, the function of UBIAD1 (MK-4 biosynthesis enzyme) and MK-4 in cartilage is unclear. In this study, we examined the function of UBIAD1 and MK-4 in chondrogenesis and differentiation using chondrogenically differentiated cells. Methods: Mouse chondrocyte progenitor ATDC5 cells were used for siRNA knockdown of UBIAD1 to determine its effects on cell proliferation and chondrogenic differentiation. Proliferation and differentiation were assessed using the WST-8 assay and Alcian blue staining, respectively. The effects of MK-4 treatment and transfection of a human UBIAD1 expression plasmid on UBIAD1 knockdown cells were also examined. RESULTS: UBIAD1 knockdown significantly decreased the proliferation and chondrogenic differentiation of ATDC5 cells. MK-4 treatment suppressed ATDC5 cell proliferation and chondrogenic differentiation. It also affected UBIAD1 knockdown ATDC5 cells during chondrogenic differentiation. However, overexpression of human UBIAD1 promoted ATDC5 cell proliferation and chondrogenic differentiation and exhibited a similar effect on UBIAD1 knockdown ATDC5 cells. These results suggest that MK-4 has an inhibitory effect on the proliferation and differentiation of UBIAD1, which exhibits a promoting effect. These results suggest that UBIAD1 and MK-4 have a role in regulating chondrocyte proliferation and differentiation and are important regulators of chondrogenic differentiation.
Vitamin K is a fat-soluble vitamin that plays an important role in blood coagulation and bone formation. It contains a 1,4-naphthoquinone skeleton and homologs depending on the side chain structure. We primarily consume vitamin K1 (phylloquinone; PK), which has a phytyl side chain, from green vegetables, and menaquinones (MK-n), which have a long isoprenoid side chain, from fermented foods.1) Menadione (MD) or vitamin K3 is a synthetic compound that lacks a side chain. Vitamin K2 (menaquinone-4; MK-4), which contains geranylgeranyl side chains, occurs at the highest concentrations in various tissues,2) because PK and MK-n are converted to MK-4 in vivo.2) Interestingly, dietary PK releases MD following cleavage of the side chain in the intestine. MD is then delivered through the mesenteric lymphatic system and blood circulation to tissues, where it is converted into MK-4 by the prenylating enzyme UbiA prenyltransferase containing protein 1 (UBIAD1), and accumulates in the form of MK-4.3) UBIAD1 is a recently discovered MK-4 biosynthetic enzyme that is localized to the endothelial reticulum4,5) Golgi complex,5,6) and mitochondria7) in a variety of tissues and cell types of vertebrates. Whether UBIAD1 exerts functions in addition to the biosynthesis of MK-4 is unknown; however, UBIAD1/ubiad1 mutations in zebrafish have been reported to cause cardiac edema and cranial haemorrhages6,8) whereas UBIAD1/heixuedian (heix) mutations in Drosophila cause defects in mitochondrial ATP production.9) In humans, UBIAD1 mutations cause a rare autosomal-dominant eye disease known as Schnyder corneal dystrophy.10) In mice, UBIAD1 is expressed in various tissues and a systemic deficiency of UBIAD1 is lethal to mouse embryos.11) Tamoxifen-dependent systemic induction of UBIAD1 deficiency is also lethal to adult mice.12)
PK, MK-n, and MK-4 act as cofactors for γ-glutamylcarboxylase (GGCX), which is involved in the post-translational modification of vitamin K-dependent proteins, such as blood coagulation factors and bone matrix proteins.13,14) MK-4 is also a ligand for the nuclear receptor steroid and xenobiotic receptor (SXR) and its murine ortholog, pregnane X receptor (PXR),15-17) which induces the expression of bone matrix proteins and activates protein kinase A (PKA) and C (PKC).18) Clinical studies have demonstrated that the administration of MK-4 prevents fractures19,20) and it has been approved for the treatment of osteoporosis in East Asian countries. Several epidemiological studies have suggested that vitamin K is associated with osteoarthritis, which is another skeletal disorder. In North America and Japan, low vitamin K intake is associated with the development of osteoarthritis.21-23) Recently, the protective effects of vitamin K signaling through SXR/PXR on articular cartilage and bone tissue were observed in PXR knockout mice. The articular cartilage in these mice was thinner and abnormal chondrogenesis occurred.24) These results suggest that MK-4 has an important role in the structure and function of cartilage tissues. UBIAD1, the enzyme that synthesizes MK-4, is expressed in all tissues and its deficiency is lethal in mouse embryos. UBIAD1 is an enzyme that synthesizes MK-4, and UBIAD1 deficiency results in the loss of MK-4. However, the effects of UBIAD1 deficiency are not completely ameliorated by MK-4 supplementation alone; thus, the presence of UBIAD1 may be important for cell function.4) SXR/PXR, the nuclear receptor for MK-4, contributes to cartilage formation, thus UBIAD1 is may also be involved in cartilage formation; however, it is unclear how UBIAD1, which is responsible for MK-4 biosynthesis in tissues, affects chondrocyte function. The function of MK-4 in chondrocytes is largely unknown. Therefore, we examined the role of UBIAD1 and MK-4 in chondrocyte proliferation and differentiation using chondrogenic ATDC5 cells.
In the present study, we knocked down UBIAD1 expression using siRNA in ATDC5 cells and evaluated the proliferative and chondrogenic differentiation potential. The results indicated that UBIAD1 knockdown reduced the proliferative and differentiation potential of ATDC5 cells. We also determined the effects of MK-4 treatment and overexpression of UBIAD1 and found that MK-4 and UBIAD1 regulate the proliferation and differentiation of chondrocytes.
ATDC5 cells (Riken Bio Resource Center Cell Bank, Ibaraki, Japan) were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/Ham’s F-12; Nacalai Tesque, Kyoto, Japan) containing 5% fetal bovine serum (Gibco, Thermo Fisher Scientific), 100 U/ml penicillin G potassium salt (Nacalai Tesque, Kyoto, Japan), and 100 μg/ml streptomycin (Nacalai Tesque, Kyoto, Japan) at 37°C in an atmosphere of 95% air and 5% CO2.
Chondrogenic DifferentiationTo induce chondrogenic differentiation, ATDC5 cells were seeded into 6-well plates at a density of 3.0 × 105 cells/well and cultured in differentiation medium containing DMEM/ F-12 with 5% FBS and 1% insulin-transferrin-selenium (10 μg/mL bovine insulin, 10 μg/mL human transferrin, 3 × 10−8 M sodium selenite; Thermo Fisher Scientific Life Sciences) at 37°C in the presence of 5% CO2. The medium was changed every 2 days.
Stealth siRNA TreatmentStealth siRNA for mouse UBIAD1, siUbiad1 (Thermo Fisher Scientific), is a 25-bp duplex oligoribonucleotide consisting of a sense strand corresponding to nucleotides 921–945 of the reported mouse Ubiad1 mRNA sequence. Alexa-labeled siRNA was used as control siRNA (Thermo Fisher Scientific). ATDC5 cells were transfected with 50 pmol of each siRNA using Lipofectamine RNAiMAX (Thermo Fisher Scientific) in 1 mL of Opti-MEM serum-reduced medium (Gibco, Thermo Fisher Scientific) based on the manufacturer’s instructions.
Transfection of Human UBIAD1 Expression PlasmidThe human UBIAD1 cDNA fragment from the pENTR221-UBIAD1 plasmid (Thermo Fisher Scientific) was subcloned into the pcDNA3.3-TOPO-TA cloning vector (Thermo Fisher Scientific) to create pcDNA3.3-UBIAD1. ATDC5 cells (2 × 105 cells/well) were cultured for 24 h and transfected with 1 µg of pcDNA3.3 (Mock) or pcDNA3.3-UBIAD1 (pUBIAD1) using Lipofectamine 2000 reagent (Thermo Fisher Scientific).
Quantitative RT-PCRTotal RNA was isolated from ATDC5 cells with Isogen (Nippon Gene) based on the manufacturer’s protocol. First strand cDNA was synthesized using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO). cDNA was mixed with TB Green® Premix Ex Taq™ II (Takara) and real-time PCR was carried out using the CFX96 real-time PCR system (Bio-Rad). Target gene mRNA levels were normalized to that of ribosomal protein lateral stalk subunit P2 (Rplp2) or 18S ribosomal RNA (18S-rRNA). The primers were as follows: mouse Ubiad1, 5′-TGAGGCGGGTTATTGAGTTGTG-3′ (forward) and 5′- GGGAAAACTGAAGACAGCAACC-3′ (reverse); human UBIAD1, 5′-CTGGCTCCTTTCTCTACACAGG-3′ (forward) and 5′- CGTAGGCGAACATCACAGCCAG-3′ (reverse); mouse Col2a1, 5′- ACGAAGCGGCTGGCAACCTCA-3′ (forward) and 5′- CCCTCGGCCCTCATCTCTACATCA-3′ (reverse); mouse Col10a1, 5′- TGCCCGTGTCTGCTTTTACTGTCA-3′ (forward) and 5′- TCAAATGGGATGGGGGCACCTACT-3′ (reverse), and mouse Rplp2, 5′- TACTAGACAGCGTGGGCATC-3′ (forward) and 5′- CAACACCCTGAGCGATGACA-3′ (reverse), mouse 18S-rRNA, 5′- GGGAGCCTGAGAAACGGC-3′ (forward) and 5′-GGGTCGGGAGTGGGTAATTT-3′ (reverse).
Cell Proliferation AssayCell morphology was observed using a phase contrast microscope (Olympus IX-70). To determine the effect of siUBIAD1 and MK-4 on cell growth, ATDC5 cells were seeded into a 96-well plate and cultured for 5 days with siUBIAD1 or various concentrations of MK-4. Cell numbers were assessed by Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan). This system utilizes WST-8 (2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt), which produces a water-soluble formazan dye upon bioreduction in the presence of an electron carrier. Cell counts were recorded at 1, 2, 3, 4 and 5 days. The wells were incubated with WST-8 solution for 3 h at 37°C and the absorbance at 450 nm was measured by spectrophotometry (iMark, Bio-Rad). The experiments were performed at least in triplicate and the results are expressed as means ± standard error.
Measurement of Chondrogenic DifferentiationChondrogenic differentiation of ATDC5 cells was assessed by staining sulfated glycosaminoglycans with Alcian blue as previously described.25) The cells were washed with PBS, fixed with 100% methanol for 20 min (room temperature), and stained with 0.1% Alcian blue 8GX (Wako, Osaka, Japan) in 0.1 M HCl overnight. After three washes with distilled water, the cells were subject to histochemical analysis. The stain was extracted with 500 µl of 6 M guanidine-HCl for 6 h at room temperature and the absorbance was measured at 620 nm by spectrophotometer.
Measurements of MK-4-d7 Converted from MD-d8 in ATDC5 CellsATDC5 cells were treated with deuterium-labeled MD (MD-d8) (1 μM). After 24 h, the cells were corrected for analysis. Deuterium-labeled MK-4 (MK-4-d7) levels were measured by LC-APCI-MS/MS as described previously.3,4,11) Note that in this study, Infinity1260 (Agilent Technology) was used for HPLC and QTRAP®4500 (SCIEX) for APCI-MS/MS.
Statistical AnalysisAll data are expressed as means ± standard error. Differences between groups were examined for statistical significance using a Student’s t-test, Dunnett’s test, or the Tukey-Kramer HSD Test. P values less than 0.05 were considered statistically significant.
The role of UBIAD1 in chondrogenesis was examined in Ubiad1 siRNA-transfected ATDC5 cells. Compared with control cells transfected with a nontargeting siRNA, one target sequence of UBIAD1 significantly decreased the expression of Ubiad1 mRNA (Fig. 1A). Cell viability was assessed using the WST8 assay (Fig. 1B) Ubiad1 knockdown decreased cell viability in a time-dependent manner and a significant decrease in cell viability was achieved after 1 day. These data indicate that UBIAD1 is required for chondrocyte proliferation.

Ubiad1 mRNA Expression and Proliferation in siUbiad1-Transfected ATDC5 Cells
(A) Ubiad1 mRNA expression in ATDC5 cells of siControl or siUbiad1-transfected ATDC5 cells after 5 days of culture. (B) Time course of cell proliferation of siControl- or siUbiad1-treated cells. Cell proliferation was measured using the WST-8 assay. Data are shown as means ± standard errors of the mean (SEM). Significant difference versus siControl-treated cells; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Student’s t-test).
The effect of Ubiad1 knockdown on chondrocyte differentiation was evaluated as the coordination of cell proliferation and differentiation are important to chondrogenesis. To determine the effect of Ubiad1 on chondrogenic differentiation, the cells were cultured in chondrogenic medium. Cartilage-specific ECM glycosaminoglycans (GAGs) were assessed by Alcian blue staining. Ubiad1 knockdown decreased GAG levels significantly (Fig. 2A). Ubiad1 knockdown cells exhibited significantly less absorbance after Alcian blue extraction compared with that of the control siRNA-transfected cells (Fig. 2B). The expression of genes associated with cartilage formation were further examined. The expression of type 2 collagen (Col2a1) and type 10 collagen (Col10a1) were measured by real-time PCR. The results indicated that Ubiad1 knockdown significantly decreased the m RNA expression of Col2a1 (Fig. 2C) and Col10a1 (Fig. 2D). Thus, Ubiad1 knockdown suppressed chondrogenic differentiation inATDC5 cells.
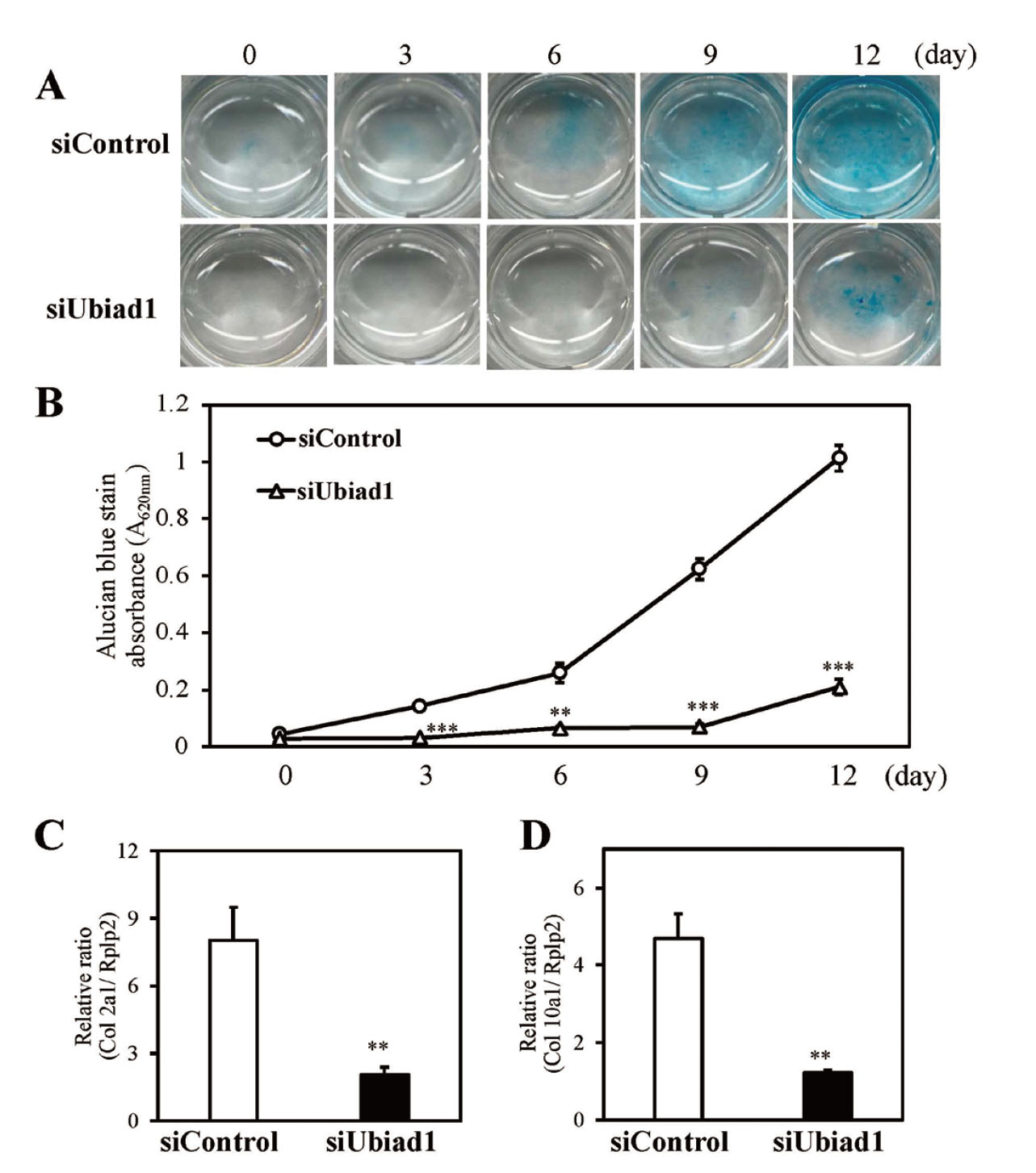
Chondrogenic Differentiation of siControl- or siUbiad1-Transfected ATDC5 Cells
(A) Representative stained culture plates are shown for the siControl or siUbiad1-transfected ATDC5 cells. (B) Absorbance measurements following Alcian blue extraction are shown as the mean ± SEM. (C) mRNA expression of type 2 collagen (Col2a1) in siControl- and siUbiad1-transfected ATDC5 cells cultured in differentiation medium on day 3. (D) mRNA expression of type 10 collagen (Col10a1) in siControl- or siUbiad1-transfected ATDC5 cells cultured in differentiation medium on day 6. Data are shown as means ± SEM. Significant difference versus siControl-treated cells; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Student’s t-test).
Ubiad1 is involved in the synthesis of MK-4. To determine whether MK-4 is associated with reduced proliferation and differentiation in ATDC5 cells following Ubiad1 knockdown, we determined the effects of MK-4 treatment. Ubiad1 knockdown reduced the proliferative potential of ATDC5 cells; however, MK-4 (0.1 µM) treatment did not prevent this reduction. Instead, MK-4 treatment significantly suppressed proliferation in control siRNA-transfected cells (Fig. 3A). The inhibitory effect of MK-4 on proliferation was dose-dependent (Fig. 3B). The effect of MK-4 treatment on chondrogenic differentiation was also evaluated. Differentiation cultures were grown in the presence and absence of MK-4 and differentiation was assessed by Alcian blue staining. The results indicated that the reduction of chondrocyte differentiation resulting from Ubiad1 knockdown was not improved by MK-4 treatment (Fig. 4A, B). At day 12 of differentiation culture, MK-4 treatment significantly reduced absorbance following Alcian blue extraction, not only in control siRNA-transfected cells, but also in Ubiad1 knockdown cells (Fig. 4B). The results suggests that MK-4 may regulate chondrogenesis in ATDC5 cells by inhibiting proliferation and differentiation.

Effect of MK-4 on Proliferation of siControl or siUbiad1-Transfected ATDC5 Cells
(A) Time course of cell proliferation of siControl- or siUbiad1-treated cells with or without MK-4 (0.1 µM). Cell proliferation was measured using the WST-8 assay. Data are shown as means ± standard errors of the mean (SEM). Significant difference versus siControl + EtOH treated cells; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Student’s t-test). (B) Dose-dependent effects of MK-4 on cell proliferation of siControl- or siUbiad1-treated cells at 5 days of culture. Data are shown as means ± SEM. Significant difference to 0 µM MK-4 in siControl-treated cells; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Dunnett’s test).

Effect of MK-4 on Chondrogenic Differentiation of siControl- or siUbiad1-Transfected ATDC5 Cells
(A) Representative stained cell culture plates are shown for siControl- or siUbiad1-transfected ATDC5 cells with or without MK-4 (0.1 µM). (B) Absorbance measurements after Alcian blue extraction are shown as the mean ± SEM. Data are shown as means ± SEM. Significant difference versus siControl + EtOH treated cells ; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Dunnett’s test). Significant difference between siUbiad + MK-4-treated cells versus siUbiad + EtOH-treated cells; # p < 0.05 (Student’s t-test).
To determine the effects of increased UBIAD1 expression on Ubiad1 knockdown ATDC5 cells, human UBIAD1 expression plasmid was transfected into UBIAD1 knockdown cells and proliferation and differentiation were evaluated. Transfection of the human UBIAD1 expression plasmid into Ubiad1 knockdown ATDC5 cells only increased human UBIAD1 expression (Fig. 5A). We examined MK-4 biosynthesis by expressed human UBIAD1. In human UBIAD1 overexpressed cells, MK-4-d7 converted from MD-d8 by human UBIAD1 was detected (Fig. 5B). Overexpression of human UBIAD1 increased proliferation in both siUbiad1 and siControl-transfected cells (Fig. 5C). The effect of human UBIAD1 expression on chondrogenic differentiation was also determined. Differentiation was evaluated by Alcian blue staining. The results indicated that a reduction of chondrocyte differentiation caused by Ubiad1 knockdown was recovered by human UBIAD1 expression (Fig. 6A and B). On day 12 of differentiation culture, human UBIAD1 significantly increased absorbance after Alcian blue extraction, not only in control siRNA-transfected cells, but also in Ubiad1 knockdown cells (Fig. 6B). Overexpression of human UBIAD1 also increased the expression of Col2a1 and Col10a1 in differentiated cultured cells (Fig. 6C and D). These results suggest that UBIAD1 acts in a promotive manner in the proliferation and differentiation of chondrocytes.

Effect of UBIAD1 Overexpression on Proliferation of siControl- or siUbiad1-Transfected ATDC5 Cells
(A) Human UBIAD1 mRNA expression in pUBIAD1 transfected siUbiad1-transfected ATDC5 cells on day 7. Significant difference between with or without pUBIAD1; *** p < 0.001 (Student’s t-test). (B) Concentration of MK-4-d7 converted from MD-d8 in pUBIAD1- and siUbiad1-transfected ATDC5 cells on day 7. Significant difference between with or without pUBIAD1; *** p < 0.001 (Student’s t-test).(C) Time course of cell proliferation of pUBIAD1 transfected siControl- or siUbiad1-treated cells. Cell proliferation was measured using the WST-8 assay. Data are shown as means ± standard errors of the mean (SEM). Significant difference versus siControl with Mock-transfected cells (siControl + Mock); * p < 0.05, ** p < 0.01 and *** p < 0.001 (Dunnett’s test). Significant difference between pUBIAD1-transfected versus Mock-transfected in siUbiad1-transfected ATDC5 cells; ## p < 0.01 and ### p < 0.001 (Student’s t-test).

Effect of UBIAD1 Overexpression on Chondrogenic Differentiation of siControl- or siUbiad1-Transfected ATDC5 Cells
(A) Representative stained cell culture plates are shown for Mock or pUBIAD1-transfected siControl or siUbiad1-transfected ATDC5 cells. (B) Absorbance measurements following Alcian blue extraction are shown as the mean ± SEM. Significant difference versus siControl + Mock-treated cells; * p < 0.05, ** p < 0.01 and *** p < 0.001 (Dunnett’s test). Significant difference between pUBIAD1-transfected and Mock-transfected in siUbiad1-transfected ATDC5 cells; # p < 0.05 (Student’s t-test). (C) mRNA expression of type 2 collagen (Col2a1) in Mock- or pUBIAD1-transfected siControl- and siUbiad1-transfected ATDC5 cells cultured in differentiation medium on day 3. (D) mRNA expression of type 10 collagen (Col10a1) in Mock- or pUBIAD1-transfected siControl- or siUbiad1-transfected ATDC5 cells cultured in differentiation medium on day 6. Data are shown as means ± SEM. There is a significant difference between different symbols (A and B); p < 0.05 (Turkey’s HSD test). Significant difference between pUBIAD1-transfected versus Mock-transfected in siUbiad1-transfected ATDC5 cells; # p < 0.05 and ### p < 0.001 (Student’s t-test).
In this study, we found that UBIAD1 expression is involved in chondrogenic differentiation in ATDC5 cells. UBIAD1 knockdown significantly reduced the proliferation and differentiation of ATDC5 cells, whereas UBIAD1 overexpression restored proliferation and differentiation. These results suggest that UBIAD1 plays an important role in chondrocyte differentiation.
UBIAD1 is involved in the MK-4 biosynthesis, lipid and cholesterol metabolism, and the maintenance of mitochondrial function. Although the effects of UBIAD1 deficiency in systemic tissues and in the pancreas have been documented, the role of UBIAD1 in chondrocytes has not been clarified. We found that UBIAD1 is expressed in chondrogenic ATDC5 cells and UBIAD1 knockdown markedly suppresses the proliferation and differentiation of ATDC5 cells. UBIAD1 is known in studies of cancer cells as transition epithelial response protein 1 (TERE1), which inhibits the proliferation of transitional cell carcinoma and prostate cancer cell lines.26-30) However, in ATDC5 cells, which exhibit chondrocyte characteristics, UBIAD1 knockdown suppressed cell proliferation and chondrogenic differentiation, whereas forced overexpression promoted proliferation and differentiation. With respect to the function of UBIAD1, Schumacher et al. reported that geranylgeranyl regulates the transport of UBIAD1 between the endoplasmic reticulum and the Golgi apparatus, and regulates cholesterol metabolism.31) Furthermore, Xu et al. found that UBIAD1 is in dynamic equilibrium between the endoplasmic reticulum and the Golgi apparatus and regulates cell proliferation.32) In cancer cells, GGPP produced by the mevalonate metabolic pathway is used for the geranylgeranylation of proteins, such as RhoA and Rac1. It has been reported that increased expression of the GGPPase enzyme, which synthesizes GGPP, promotes cancer cell proliferation.33) UBIAD1 is a key regulator of MK-4 synthesis and increased expression of UBIAD1 in cancer cells may result in the use of GGPP as a substrate for MK-4 synthesis, resulting in growth inhibition. In contrast, in normal cells, UBIAD1 expression likely contributes to the maintenance of cell viability. Zebrafish UBIAD1 mutants exhibit abnormalities in heart and vascular endothelial cells, which are ameliorated by the expression of human UBIAD1.34) Thus, UBIAD1 exhibits distinct functions in normal cells versus cancer cells. In chondrocytes, it regulates chondrocyte function and promotes chondrogenesis through proliferation and differentiation.
In the present study, we observed that MK-4 treatment did not enhance the proliferation or differentiation capacity of ATDC5 cells, in which UBIAD1 expression was knocked down. Thus, the presence of UBIAD1 itself is important for chondrocyte function. In contrast, MK-4 suppressed the proliferation and differentiation of ATDC5 cells. MK-4 induces the expression of bone matrix proteins via SXR/PXR in osteoblasts, promotes neuronal differentiation in neural stem cells,35) and protects against oxidative stress in neurons.36) MK-4 can also rescue transient global cerebral ischemia/reperfusion consequences through its anti-inflammatory and antioxidative stress features in rat.37) However, MK-4 also inhibits proliferation and induces apoptosis in cancer cells. Thus, the function of MK-4 varies among cell types, such as normal and cancer cells. In the present study, MK-4 inhibited the proliferation and differentiation of ATDC5 cells at concentrations of 0.1 µM or higher, which suggests that at high concentrations, it inhibits the proliferation and differentiation of chondrocytes. In other words, the presence of UBIAD1 itself is essential for the proliferation and differentiation of chondrocytes, whereas MK-4 synthesized by UBIAD1 may have a negative regulatory role in preventing the excessive proliferation and differentiation of chondrocytes.
Recently, it was reported that individuals with high vitamin K intake have a lower risk of developing osteoarthritis and that vitamin K is effective against osteoarthritis.38) In osteoarthritis, there is an increase in Col10a1 expression in articular cartilage tissues because of an increase in hypertrophic cartilage.39-41) Our data suggest that vitamin K intake may suppression the development of osteoarthritis through MK-4 biosynthesis by UBIAD1 in cartilage tissue and the proper regulation of chondrocyte differentiation by MK-4. Because a direct relationship between MK-4 and UBIAD1 as well as osteoarthritis and its mechanism of action are unclear, we will analyze the effects of vitamin K and UBIAD1 in mouse models of osteoarthritis in the future.
In conclusion, UBIAD1 regulates chondrocyte cell proliferation and differentiation, and may function as an important factor in chondrocytes. MK-4 may be a negative regulator of proliferation and differentiation in chondrocytes. The results of this study suggest that UBIAD1 and MK-4 contribute to the maintenance of normal chondrogenesis and prevent the development of diseases, such as osteoarthritis. However, the underlying mechanism of action of UBIAD1 and MK-4 function in chondrocyte differentiation requires further study. Therefore, we intend to develop mouse disease models and chondrocyte-specific UBIAD1-deficient mice to examine the role of UBIAD1 and MK-4 in cartilage diseases, such as osteoarthritis.
We would like to thank Enago (www.enago.com) for English language editing. This research was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP21K06554 (K.N.).
Conflict of interestThe authors declare no conflict of interest.