Abstract
Gummy drugs are dried jelly formulations prepared by adding a gelling agent to saccharides, which are then cooled and solidified. Epinastine hydrochloride (Epi) is commonly used to treat allergic diseases as a prescription and over-the-counter drug. However, the extremely bitter taste of Epi would affect its acceptability among patients. In this study, we aimed to improve the palatability of a gummy drug containing Epi (Epi-G) via organoleptic masking. Epi-G (10 mg of Epi/3.5 g of gummy drug) with two different organoleptic masking formulations, namely aspartame, cocoa powder, and chocolate flavoring (C-Epi-G) or aspartame, L-menthol, and lemon flavoring (L-Epi-G). The gustatory sensation test included six healthy adult volunteers (23.3 ± 1.8 years). We used a visual analogue scale (VAS) to evaluate bitterness, sweetness, and the overall palatability of each Epi-G formulation during chewing and after spitting out the drugs. In the gustatory sensation test, the VAS scores for bitterness and sweetness were decreased and increased for C-Epi-G and L-Epi-G, respectively, compared with the values for Epi-G without organoleptic masking. The VAS scores for overall palatability during chewing for C-Epi-G and L-Epi-G were significantly increased by 2.3- and 2.0-fold, respectively, versus the value for Epi-G. The score after spitting out C-Epi-G remained higher than that of Epi-G. These data illustrated that Epi-G with organoleptic masking had good palatability, which could improve patient adherence to treatment. The gummy drugs could represent an alternative dosing formulation for pediatric and geriatric patients by allowing them to take the drugs more easily than other oral formulations.
INTRODUCTION
In pharmacotherapy, the medication adherence of patients is critical to ensure that the expected therapeutic effects are obtained.1-3) We have been conducted research to develop “gummy drugs” based on gummy beads as new formulations to enhance the dosing of medicines.4-6) Gummy drugs are dried jelly formulations prepared by adding a gelling agent to saccharides, which are then cooled and solidified. The method of manufacturing gummy drugs is simple, requiring no special equipment, and it could permit the ability to prepare gummy drugs as in-hospital preparations or in-pharmacy formulations according to the individual needs of each patient. In addition, gummy drugs can be easily ingested and swallowed, as they do not require water for consumption and they can be chewed easily. Therefore, gummy drugs are expected to improve adherence, in especially pediatric and geriatric patients, who often have difficulty swallowing oral drugs. We have previously prepared gummy drugs containing bitter-tasting acetaminophen, aripiprazole, and amenamevir.6-9)
Because the unpleasant taste and poor palatability of drugs generally result in poor adherence, it is important to develop pharmaceutical formulations to overcome these issues. Taste-masking methods including physical, chemical, and organoleptic masking methods are used to mask the unpleasant tastes of drugs, and they play an important role in dosage formulations. Organoleptic masking methods, including the addition of sweeteners and flavoring agents, are easy and effective strategies for masking, and commonly used formulations include syrup formulations, powder formulations, and orally disintegrating tablets (ODTs).10-13) However, few reports have described the effects of organoleptic masking on the palatability of gummy drugs.
Epinastine hydrochloride is a second-generation antihistamine and antiallergic drug. It is commonly used for conditions such as allergic rhinitis as a prescription or over-the-counter (OTC) formulation. This drug has an extremely bitter taste, which could affect its palatability among patients.14,15) Pharmaceutical companies are developing formulations that use masking technology.16) However, the bitter taste derived from epinastine hydrochloride may prevent pediatric patients from taking the drug.
In this study, we aimed to develop gummy drugs containing epinastine hydrochloride with preferential palatability via organoleptic masking. We prepared gummy drugs containing epinastine hydrochloride with two different masking formulations and evaluated the effects of organoleptic masking on the palatability of gummy drugs using gustatory sensation tests in healthy volunteers.
MATERIALS AND METHODS
Materials
Gelatin (AP-50), hydrogenated maltose starch syrup (Amalty syrup), and D-sorbitol (75% oral solution) were purchased from Nippi, Inc., (Tokyo, Japan), Mitsubishi Shoji Foodtech Co., Ltd. (Tokyo, Japan), and Kowa Company, Ltd. (Nagoya, Japan), respectively. All other samples were commercially obtained as follows: L-menthol (Nagaoka Co., Ltd., Hyogo, Japan), aspartame (Ajinomoto Pharmaceutical Co., Ltd., Tokyo, Japan), cocoa powder (NF-15; Morinaga Shoji Co., Ltd.), flavors (lemon and chocolate, Takasago International Co., Ltd., Tokyo, Japan), and flavoring (Takasago International Co., Ltd.). Other chemicals used were of regent grade.
Preparation of Gummy Drugs Containing Epinastine Hydrochloride
Gummy drugs were prepared as described previously with some modifications.4,5) According to previous findings,5) the most preferred placebo gummy formulation was confirmed by human sensory tests (6.9% gelatin, 46.7% hydrogenated maltose starch syrup, and 26.6% D-sorbitol solution). The formulation is based on this previous study and is set at approximately the same proportions (Table 1). We prepared a gummy drug without masking (Epi-G) and two gummy drugs with different organoleptic masking formulations (C-Epi-G and L-Epi-G, Table 1). Amalty syrup and the D-sorbitol solution were mixed and heated (up to 135°C) to concentrate the solution. Separately, gelatin was dissolved in purified water by heating at approximately 60°C. The solutions were then mixed and kept at 70°C. Then, the solution of powdered epinastine hydrochloride tablets and citric acid suspended in water were added in sequence at a temperature of 70°C. The tablet powder was dispersed well in the mixture solution to uniformly obtain the drug content (10 mg/3.5 g of formulation). For the epinastine gummy drugs with organoleptic masking, aspartame, cocoa powder, and chocolate flavoring (C-Epi-G) or aspartame, L-menthol, and lemon flavoring (L-Epi-G) were added as masking agents. Finally, 3.5 g of each mixture were dispensed into a plastic plate shaped like a round pocket using a syringe and cooled for 24 h at room temperature.
Table 1. Formulation of Gummy Drugs Containing Epinastine for Clinical Study
| Component(%) |
Epi-G |
C-Epi-G |
L-Epi-G |
| Gelatin |
6.9 |
6.9 |
6.9 |
| Hydrogenated maltose starch syrup |
47.1 |
47.1 |
47.1 |
| D-Sorbitol sol. |
26.8 |
26.8 |
26.8 |
| Citric acid |
0.7 |
0.7 |
0.7 |
| Epinastine |
0.03 |
0.03 |
0.03 |
| Aspartame |
- |
0.8 |
1.0 |
| Cocoa powder NF-15 |
- |
1.0 |
- |
| Chocolate flavor |
- |
0.1 |
- |
| L-menthol |
- |
- |
0.1 |
| Lemon flavor |
- |
- |
0.1 |
| Water |
18.1 |
16.4 |
17.0 |
| Overall |
100 |
100 |
100 |
Measurements of the Penetration Distance of the Gummy Drugs
We measured the penetration distance of the gummy drugs to evaluate their hardness. The penetration distance of each gummy drug formulation was measured using a penetrometer (Ikemoto Scientific Technology Co., Ltd., Tokyo, Japan). The needle tip of the instrument was placed in contact with the surface of each gummy drug, and then a 50-G needle holder was used to penetrate the gummy drug with the needle for 5 s.
Gustatory Sensation Test
A gustatory sensation test was conducted for Epi-G, C-Epi-G, and L-Epi-G with six healthy volunteers (three men and three women; mean age = 23.3 ± 1.8 years), who participated in the study after providing written informed consent. The study protocol was approved by the Ethics Committee of the Hamamatsu University School of Medicine (R15-172). The gustatory sensory test was conducted in a randomized crossover, single-blind trial consisting of one stage that tested the taste of gummy drugs.
In the trial, the bitterness, sweetness, and overall palatability of the gummy drugs were evaluated using a 100-mm visual analogue scale (VAS) by placing a mark along a 100-mm line. The schedule of this trial is presented in Fig. 1. First, volunteers placed the gummy drug in their mouths without blindfold and chewed for 20 s. They evaluated bitterness (0: not bitter at all, 100: very bitter), sweetness (0: not sweet at all, 100: very sweet), and overall palatability (0: very bad, 100: very good) while chewing a gummy (first evaluation). All volunteers spat out the chewed gummy and rinsed their mouths out with water immediately. Then, all volunteers were asked to complete the VAS again (second evaluation). The different gummy drugs were tested in 30-min intervals.
Statistical Analyses
All data are expressed as the mean ± SD. Statistical analysis was performed using a paired t-test with the post-hoc Bonferroni correction using GraphPad Prism software (version 5.0; GraphPad-San Diego, CA, USA). A significant difference compared with unmasked Epi-G was identified by a p-value smaller than 0.025 via Bonferroni correction using a paired t-test.
RESULTS
Appearance and Penetration Distance of Gummy Drugs
The appearance of the prepared gummy drug is presented in Fig. 2. Epi-G was white and transparent, whereas C-Epi-G and L-Epi-G were brown and white, respectively. The penetration distances of Epi-G, C-Epi-G, and L-Epi-G were 10.1, 9.3, and 10.3 mm, respectively.
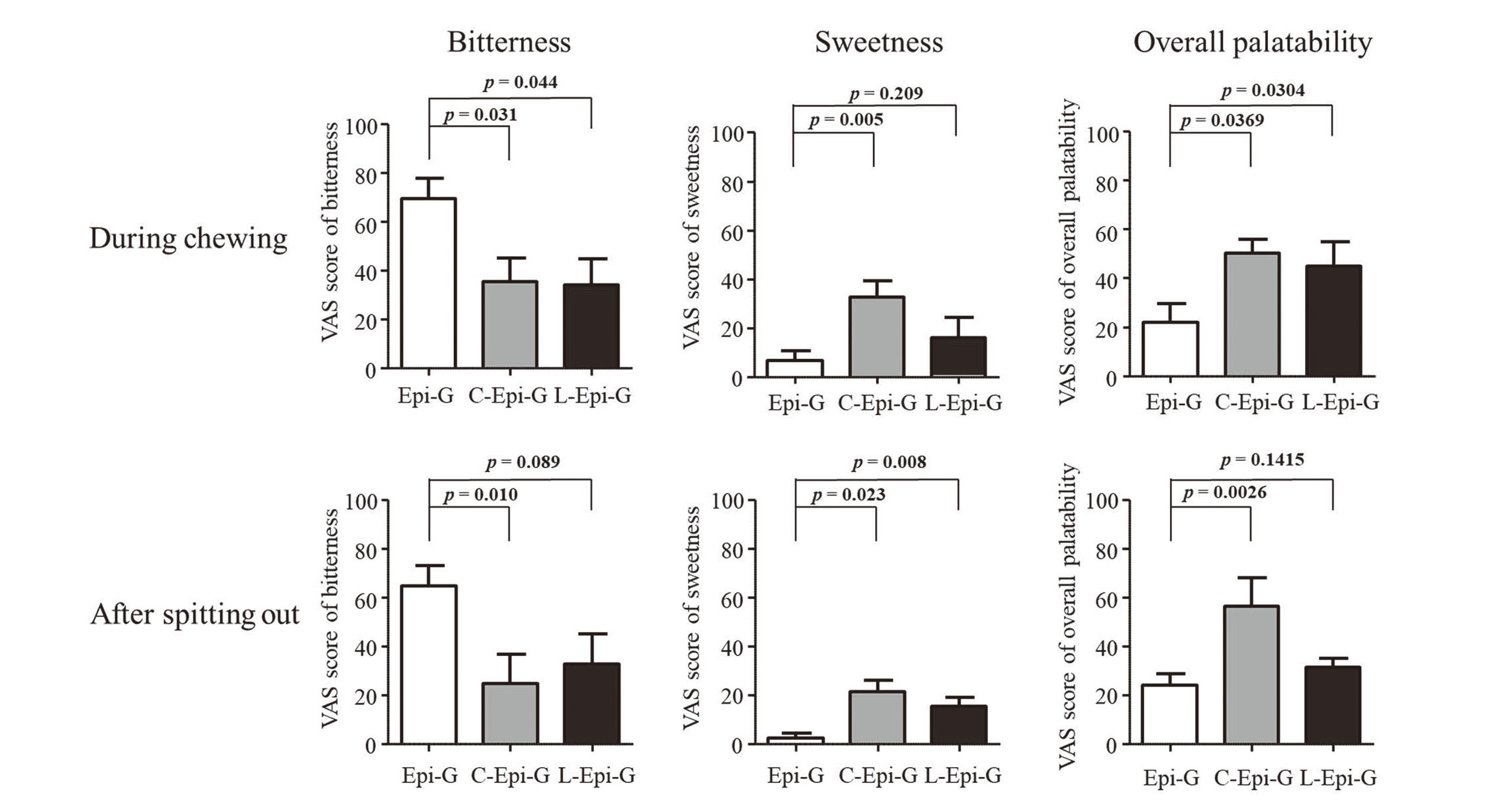
VAS Scores for Bitterness, Sweetness, and Overall Palatability for the Gummy Drugs in the Gustatory Sensation Test
To evaluate the masking of the unpleasant taste of the drug, the VAS scores of C-Epi-G and L-Epi-G were compared for each taste parameter (Fig. 3).
The VAS score of Epi-G for bitterness was 70 during chewing, and the score remained at a similar level (VAS = 65) after participants spit out the drug. Compared with Epi-G, C-Epi-G and L-Epi-G had 62% and 50% lower VAS scores, respectively. In addition, the VAS scores for bitterness after spitting out C-Epi-G and L-Epi-G were lower than that of Epi-G (25 and 33, respectively; p = 0.010 and 0.089, respectively).
The VAS scores for sweetness of C-Epi-G and L-Epi-G during chewing were 33 and 16, respectively, which were 3.9- and 1.4-fold higher than that of Epi-G, respectively (Fig. 3). After spitting out the formulations, the VAS scores were 21.3 and 15.3 for C-Epi-G and L-Epi-G, respectively, representing 7.5- and 5.1-fold increases over that for Epi-G, respectively.
Overall palatability can be defined as an overall assessment of the acceptability of taste and mouthfeel. The overall palatability of the gummy drugs was also evaluated using VAS (Fig. 3). The VAS scores for overall palatability during chewing were 50.2 and 45.0 for C-Epi-G and L-Epi-G, respectively, which were 2.3- and 2.0-fold higher than that of Epi-G, respectively. After spitting out the compounds, the VAS score for C-Epi-G was 2.3-fold higher than that of Epi-G (p = 0.0026).
DISCUSSION
In this study, we aimed to develop gummy drugs containing epinastine hydrochloride with improved palatability using organoleptic masking. The gummy drug contained 10 mg of epinastine per gummy, which is the same content as the prescription formulation.
We selected two different organoleptic masking formulations for the epinastine gummy drugs, namely cocoa-flavored and menthol- and lemon-flavored gummy drugs, which were expected to taste sweet and cool, respectively. The penetration distance of the gummy drugs ranged from 9.3 to 10.3 mm, in line with clinically preferred physical characteristics for gummy drugs in a previous report.5) Thus, it was considered that the gummy drugs prepared in this study have appropriate physical properties for clinical use.
The VAS score for bitterness of Epi-G, which lacked organoleptic masking, was high both during chewing and after spitting out the gummy (70 and 65, respectively), suggesting that the strong bitter taste of epinastine in Epi-G was noticed by volunteers both during and after chewing the gummy. Epinastine hydrochloride is highly water-soluble, and thus, the drug dissolves quickly in saliva in the oral cavity during chewing and features a strong bitter taste.
Organoleptic masking methods effectively decreased the bitterness of epinastine and improved its overall palatability. It has been reported that the bitterness of a drug can be masked using several methods. One taste-masking method involves suppressing the bitterness of a drug by modifying its organoleptic properties by adding a flavoring substance or bitter blocker to the preparation.10-13,17) In C-Epi-G with cocoa-flavored masking, the VAS score of bitterness was reduced, and that of sweetness was increased markedly. The VAS score of overall palatability for the cocoa-flavored formulation was more than 2-fold higher than that of Epi-G both during and after chewing. This indicates that the addition of cocoa powder and a sweetener effectively improves the palatability of the drug. Cocoa powder is typically used to mask the bitterness of foods and medicines. It was reported that the bitter taste of rebamipide was suppressed in combination with cocoa powder.12) It was reported that the oil component in cocoa powder effectively suppressed bitterness and that the flavor of cocoa powder itself also contributes to the improved palatability. Previous reports on the effect of cocoa powder on palatability focused only on ODTs, but our study found that addition of cocoa powder to gummy drugs effectively suppressed bitterness.12,18)
Compared with Epi-G, L-Epi-G containing lemon flavoring, L-menthol, and a sweetener featured a reduced VAS score for bitterness, and its overall palatability during chewing was similar as that of C-Epi-G. Sugiura et al. revealed that the combination of a sweetener and double-mint flavoring, which has a similar sensation as L-menthol, enhanced sweetness and improved the overall palatability of famotidine ODTs.19,20) In addition, Sotoyama et al. indicated that the bitter taste of olopatadine hydrochloride, another other antihistamine agent, in ODTs was suppressed by citric acid, and ODTs containing the combination of citric acid, yogurt flavoring, and aspartame were the most suitable formulations regarding palatability.21) Therefore, it is likely that the sour and cool taste of L-Epi-G improved its palatability. Conversely, the VAS score after spitting out the gummy drug was only 1.2-fold higher than that of Epi-G, indicating that the beneficial effect on palatability in L-Epi-G was not retained after spitting out the drug. Thus, L-menthol could have a short duration of action. Moreover, storage tests were conducted on similar formulations with sensory masking (C-Epi-G and L-Epi-G, data not shown) at 40°C for 6 months. As a result, it was confirmed that the dissolution of epinastine was not affected and no significant change in content (approximately 90%) was observed. Furthermore, it was confirmed that there was no change in the appearance of the gummy drugs during storage and that there was no mold contamination.
One limitation in this study was the small sample size in the gustatory sensation test, and the sample size was insufficient for calculating statistically significant differences. In addition, the volunteers enrolled in this study were all healthy young adults. Thus, further studies containing larger numbers of subjects from various populations including patients are required to clarify the impact of taste-masking agents on the palatability of epinastine gummy drugs.
Conclusion
We developed gummy drugs containing epinastine with good palatability through the inclusion of several organoleptic masking agents that would be acceptable to most patients. The use of gummy formulations of medicinal drugs allows infants and geriatric patients to swallow the drugs more easily via chewing and improves the palatability of the agents compared with that of other oral formulations. Therefore, gummy drugs might improve patient adherence to medication. Alternatively, OTC medicines can be taken as needed, making them convenient for modern life. We therefore believe that gummy drugs represent an attractive formulation for both prescription and OTC drugs.
Acknowledgments
We are grateful to Ms. Haruka Nakagawa and Mr. Shuta Seki for excellent technical assistance. S.T. is supported by a Grant-in-Aid for Scientific Research (21K15298C0). We thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1) Cleemput I, Kesteloot K. Economic implications of non-compliance in health care. Lancet, 359, 2129–2130 (2002).
- 2) Baguley D, Lim E, Bevan A, Pallet A, Faust SN. Prescribing for children - taste and palatability affect adherence to antibiotics: a review. Arch. Dis. Child., 97, 293–297 (2012).
- 3) Cram A, Breitkreutz J, Desset-Brèthes S, Nunn T, Tuleu C; European Paediatric Formulation Initiative. (EuPFI). Challenges of developing palatable oral paediatric formulations. Int. J. Pharm., 365, 1–3 (2009).
- 4) Uchida S, Hiraoka S, Namiki N. Development of gummi drugs of aripiprazole as hospital formulations. Chem. Pharm. Bull. (Tokyo), 63, 354–360 (2015).
- 5) Nakagaki F, Uchida S, Tanaka S, Namiki N. Palatability and Preference of Gummi Formulations with Various Pharmaceutical Characteristics. Chem. Pharm. Bull. (Tokyo), 66, 452–457 (2018).
- 6) Namiki N, Takagi N, Yuasa H, Kanaya Y. Stability of gummy drug under storage. Yakuzaigaku, 57, 86–94 (1997).
- 7) Namiki N. Formulation development for the purpose of improving medication adherence. Yakugaku Zasshi, 141, 1173–1184 (2021).
- 8) Umemoto Y, Tanaka S, Kambayashi A, Sugimoto K, Kashiwagura Y, Namiki N, Uchida S. Gummi formulations comprising amenamevir solid dispersions with polyvinyl alcohol. Chem. Pharm. Bull. (Tokyo), 69, 862–871 (2021).
- 9) Kawamoto S, Tanaka S, Miura M, Kashiwagura Y, Kamiya C, Hakamata A, Odagiri K, Inui N, Watanabe H, Namiki N, Uchida S. Palatability of aripiprazole gummies prepared from commercially available products: pharmaceutical formulation for improving patient adherence. Chem. Pharm. Bull. (Tokyo), 71, 441–446 (2023).
- 10) Nakano Y, Maeda A, Uchida S, Namiki N. Preparation and evaluation of unpleasant taste-masked pioglitazone orally disintegrating tablets. Int. J. Pharm., 446, 160–165 (2013).
- 11) Matsui R, Uchida S, Namiki N. Combination effect of physical and gustatory taste masking for propiverine hydrochloride orally disintegrating tablets on palatability. Biol. Pharm. Bull., 38, 17–22 (2015).
- 12) Takano H, Uchida S, Kashiwagura Y, Tanaka S, Hakamata A, Odagiri K, Inui N, Watanabe H, Namiki N. Preparation of cocoa powder-containing orally disintegrating tablets of rebamipide (rebamipide chocolet) and evaluation of their clinical palatability. Chem. Pharm. Bull. (Tokyo), 67, 112–119 (2019).
- 13) Rachid O, Simons FE, Rawas-Qalaji M, Simons KJ. An electronic tongue: evaluation of the masking efficacy of sweetening and/or flavoring agents on the bitter taste of epinephrine. AAPS PharmSciTech, 11, 550–557 (2010).
- 14) Sonal D. FDA clinical review (2009). https://www.fda.gov/media/77858/download
- 15) Ito M, Ikehama K, Yoshida K, Haraguchi T, Yoshida M, Wada K, Uchida T. Bitterness prediction of H1-antihistamines and prediction of masking effects of artificial sweeteners using an electronic tongue. Int. J. Pharm., 441, 121–127 (2013).
- 16) Suzuki T, Okuda Y, Okimoto K. Formulation Design of Epinastine hydrochloride DS for pediatric 1% “TOWA. J. Pharm. Sci. Technol. Jpn, 74, 180–186 (2014).
- 17) Hashimoto Y, Matsunaga C, Tokuyama E, Tsuji E, Uchida T, Okada H. The quantitative prediction of bitterness-suppressing effect of sweeteners on the bitterness of famotidine by sweetness-responsive sensor. Chem. Pharm. Bull. (Tokyo), 55, 739–746 (2007).
- 18) Kondo C, Fukuoka E, Sasaki T, Namiki N, Takano H, Yasumuro O, Yamamoto T. Development of Chocolate Flavored Oral Rapidly Disintegrating Tablets (Chocolets): Optimal Conditions in Preparing Extemporaneous Formulation of Rebamipide Chocolets. J. Pharm. Sci. Technol. Jpn, 67, 347–355 (2007).
- 19) Sugiura T, Uchida S, Namiki N. Taste-masking effect of physical and organoleptic methods on peppermint-scented orally disintegrating tablet of famotidine based on suspension spray-coating method. Chem. Pharm. Bull. (Tokyo), 60, 315–319 (2012).
- 20) Tokuyama E, Matsunaga C, Yoshida K, Mifsud JC, Irie T, Yoshida M, Uchida T. Famotidine orally disintegrating tablets: bitterness comparison of original and generic products. Chem. Pharm. Bull. (Tokyo), 57, 382–387 (2009).
- 21) Sotoyama M, Uchida S, Tanaka S, Hakamata A, Odagiri K, Inui N, Watanabe H, Namiki N. Citric Acid Suppresses the Bitter Taste of Olopatadine Hydrochloride Orally Disintegrating Tablets. Biol. Pharm. Bull., 40, 451–457 (2017).