2023 Volume 6 Issue 6 Pages 209-216
2023 Volume 6 Issue 6 Pages 209-216
Ethanol, a widely used toxic chemical in human cultures, exerts its toxicity throughout the human body, with the nervous system being particularly vulnerable, leading to various neurological disorders. Despite the prevalence of peripheral neuropathies associated with alcohol abuse, the causal mechanism of ethanol-induced peripheral neuropathies remains largely unknown. This study investigated the impact of repeated ethanol intake on the histological features of trigeminal ganglia in mice. Histological analysis revealed that blood vessels in the trigeminal ganglia exhibited distinct morphological and molecular characteristics based on their location, specifically in regions where neuronal cell bodies or axonal fibers predominate. Administration of a binge level of ethanol for four consecutive days induced significant blood vessel remodeling in both regions. Unexpectedly, the morphology of trigeminal neurons and their axons remained unaffected by ethanol. Given the observed suppression of IBA1/AIF1-positive microglia-like cells by ethanol, we explored whether manipulative suppression of microglia-like cell activity with minocycline could recapitulate ethanol-induced effects. Remarkably, administration of minocycline, instead of ethanol, resulted in similar blood vessel remodeling, suggesting that the repeated intake of a binge level of ethanol causes blood vessel remodeling through the suppression of microglia-like cells in the trigeminal ganglia. These findings provide valuable insights into the complex interplay between ethanol exposure, microglia-like cell activity, and vascular changes in the trigeminal ganglia.
Alcohol, an umbrella term for substances where a hydroxy group replaces a hydrogen atom in hydrocarbons, is colloquially associated with ethyl alcohol, or ethanol, in everyday language. Despite its widespread use, ethanol, highly toxic yet utilized without precise awareness of its dangers, has dual effects on the human body—eliciting feelings of euphoria and well-being while exerting acute and chronic toxicity, heightening the risk of neuronal disorders. Alcoholism patients exhibit diverse neurological symptoms, including cognitive disorders affecting central and peripheral systems, with peripheral neuropathies prevalent in over 40% of chronic alcohol abusers.1) These alcohol-related neuropathies are heterogenous in nature and present diverse clinicopathological features.2) They primarily result from axonal degeneration but can also manifest due to demyelination and functional impairment without evident structural changes.3,4) Even at low intoxicating doses, ethanol diminishes sensory responses in noradrenaline-containing neurons within the locus coeruleus, making the sensory system a key target for ethanol toxicity.5) Although the toxic mechanisms of ethanol on the brain have been intensely investigated, the ways in which ethanol damages and induces dysfunction of the peripheral nervous system remain elusive.
The cardiovascular system is also susceptible to ethanol toxicity.6,7) Acute exposure to ethanol elevates blood pressure through reducing endothelium-dependent blood vessel relaxation, which involves increased responsiveness of the renin-angiotensin system and sensitivity of endothelial cells to nitric oxide.8,9) Chronically, ethanol abuse enhances vascularization, leading to local vascular remodeling.10,11) Likewise, repeated ethanol consumption causes oxidative stress and inflammatory cytokine secretion in blood vessel endothelial cells.12) These long-term vascular changes contribute to the pathogenesis of hypertension as well as cardiac neuropathies.13,14) Given the diverse regulatory and toxic effects of ethanol on blood vessels, depending on location and intake patterns, a meticulous examination of ethanol toxicity is imperative for a comprehensive understanding of the biological consequences of inappropriate ethanol intake.15)
Microglia, a type of glial cells in the central nervous system and integral in regulating brain blood vessels, are sensitive to ethanol and mediate its neurotoxicity through the production of inflammatory cytokines and reactive oxygen species as well as increased glutamate secretion.16,17) Microglial responses to ethanol vary based on regional localization and exposure conditions, mirroring ethanol’s effects on blood vessels.18) In the trigeminal ganglia, glial cell composition differs from the central nervous system. Astrocyte is absent in the trigeminal ganglia and is functionally compensated by glial fibrillary acidic protein-positive satellite cells. Besides, like the brain and spinal cord, there are populations of cells positive for immunoreactivity to ionized calcium-binding adaptor molecule 1 (IBA1)/allograft inflammatory factor 1 (AIF1) antibody, a marker for active microglia.19,20) IBA1/AIF1, however, is also expressed in macrophages in the periphery, therefore the exact cell type of the IBA1/AIF1-positive population in the trigeminal ganglia is still in debate.21,22) We refer to these IBA1/AIF1-positive cells in the trigeminal ganglia as “microglia-like cells” in this manuscript. These microglia-like cells in the trigeminal ganglia may be responsible for the modulation of sensory signal transmission. 21,22)
To unveil the pathological effects and mechanisms of ethanol toxicity, our investigation has explored the impact of excess ethanol intake on the nervous system, revealing blood vessel remodeling in the mouse cerebral cortex.23) However, it remains uncertain whether blood vessels in other nervous system regions undergo similar remodeling due to excess ethanol intake. This manuscript addresses this gap by examining the morphological features of blood vessels in the trigeminal ganglia under ethanol influence.
Male ICR mice (10–14 weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). Mice were individually housed at the animal facility at Kobe Pharmaceutical University and allowed to acclimatize for at least one week before the start of the experiments. All procedures involving the animal experiments in this study were conducted following the Guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan. The protocols were approved by the Kobe Pharmaceutical University Committee for Animal Care and Use.
Ethanol, at 2 g/kg body weight (B.W.)/day as 20% v/v saline solution, or minocycline, at 45 mg/kg B.W./day, was intraperitoneally administered daily for four consecutive days. Reagent doses followed previous manuscripts.23-25) For the control mice, an equivalent amount of saline was intraperitoneally injected. Analysis was performed for two days after the final administration of reagents.
ImmunohistochemistryMice were deeply anesthetized with isoflurane, and 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) was transcardially perfused. The brain was fixed in 4% PFA in PBS at 4°C for 6 h and cryoprotected in 30% sucrose in PBS at 4°C overnight. The 30-μm horizontal sections of the trigeminal ganglia were prepared using a cryostat (SLEE medical GmbH, Mainz, Germany).
Immunohistochemistry was performed as described in our previous manuscript, with slight modifications.26) Tissue sections were fixed in 4% PFA in PBS at room temperature for 5 min, briefly washed with PBS, and incubated in 10 mM Tris buffer (pH 9.0) at 75°C for 40 min for antigen retrieval. They were then washed in PBS three times, blocked with 1.5% fetal bovine serum (FBS) in PBS, and incubated with rabbit antibodies against CD31 (AF3628, R&D Systems, Inc., Minneapolis, MN, USA), CD34 (ab81289, Abcam, plc., Cambridge, UK), VEGFR1 (ab2350, Abcam), VEGFR2 (#2479, Cell Signaling Technology, Inc., Danvers, MA, USA), IBA1/AIF1 (019-19741, FujiFilm Wako Pure Chemical, Co., Osaka, Japan), Homer1 (ab184955, Abcam), or PGP9.5 (ab27053, Abcam) at 4°C overnight. The sections were then washed with PBS and incubated with Alexa488- or 568-conjugated anti-rabbit IgG, or Alexa488-conjugated anti-goat IgG (Jackson ImmunoResearch, West Grove, PA, USA) diluted in PBS containing 1.5% FBS together with 4′,6-diamidino-2-phenylindole (DAPI), at room temperature for 3 h. They were washed in PBS and mounted with Fluoromount-G (SouthernBiotech, Birmingham, AL, USA).
All images were captured using an Axio Scope A1 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and processed with the GNU Image Manipulation Program (GIMP), ver. 2.10.32., an open source software for manipulating images. The length of immunoreactive signals was measured using ImageJ software. Calculated results were expressed as mean ± standard deviation. Data were statistically analyzed by Student’s t-test using Excel software.
In the trigeminal ganglia, neurons form clusters according to their projection areas, ophthalmic, maxillary, and mandibular regions (Fig. 1A). We refer to these areas with neuronal clusters as the “neuronal cell body region.” The areas outside of neuronal regions are mainly formed by neuronal axons and surrounding Schwann cells, referred to as the “fibrous region” in the following part. To visualize blood vessels in the trigeminal ganglia, CD34 was used as a marker for blood vessels. CD34 is a transmembrane glycoprotein found on vascular endothelial cells and hematopoietic progenitor cells.27) Immunostaining with the anti-CD34 antibody visualized blood vessels in both neuronal cell body and fibrous regions with different shapes (Fig. 1B). In the neuronal cell body region, CD34-positive cells formed a tube-like structure (Fig. 1Ba and Bb). In contrast, CD34-positive signals were found as both tube-like and line-like structures in the fibrous regions (Fig. 1Bb and Bc). The line-like CD34 signals might be microvessels but some of them may be the axons of trigeminal neurons, as a previous study implicated that anti-CD34 antibody stains peripheral axons.28)
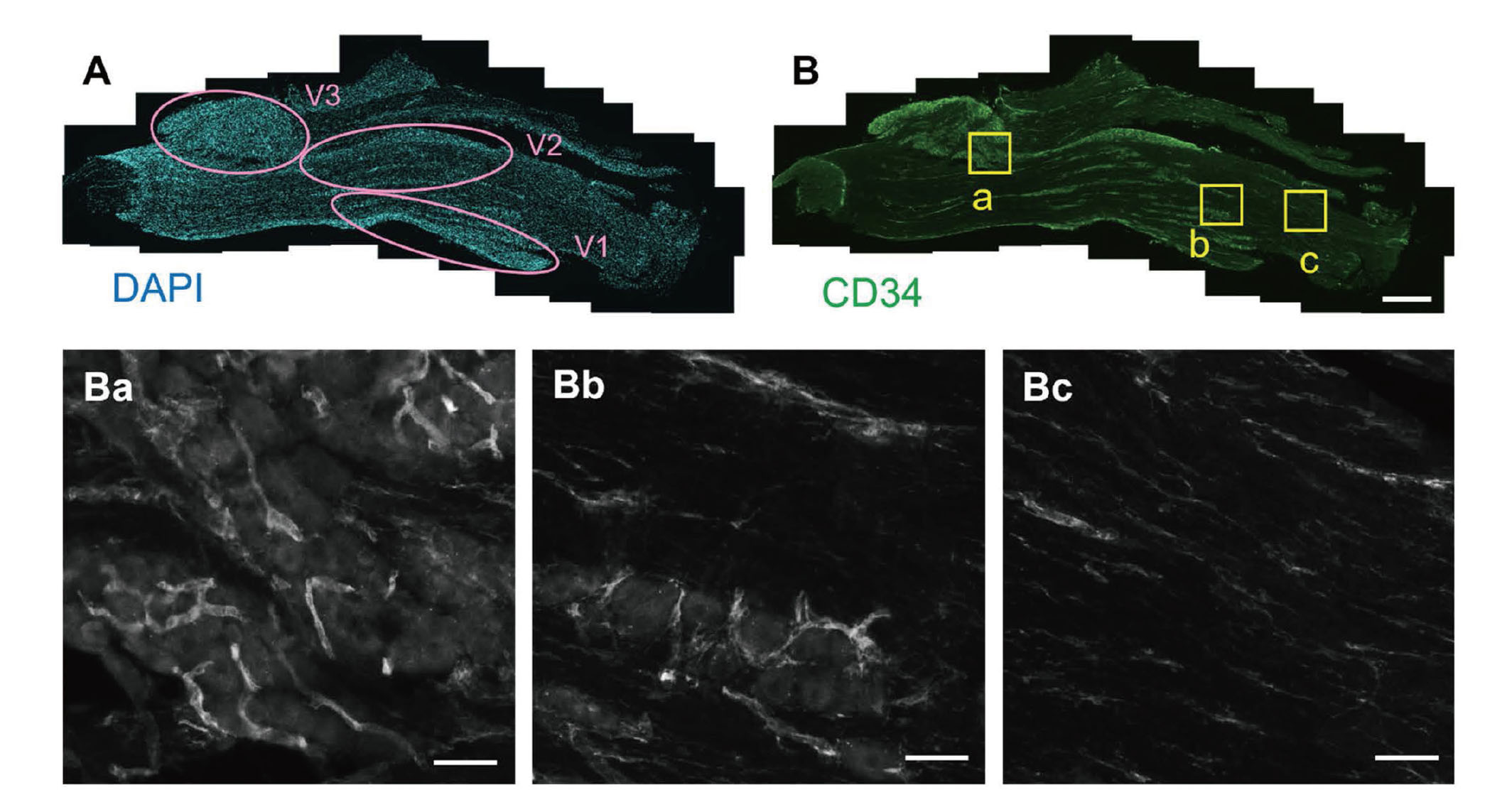
Blood Vessel Structure in the Trigeminal Ganglia
The mouse trigeminal ganglia sections were stained with DAPI (A) or anti-CD34 antibody (B). The yellow square regions in panel B are enlarged in panels Ba (neuronal cell body region), Bb (the region with both neuronal cell body and fibrous axons), and Bc (fibrous region). V1, ophthalmic division; V2, maxillary division; V3, mandibular division. Scale bars: 500 μm (B), 50 μm (Ba, Bb, Bc).
To reveal the molecular characteristics of the trigeminal blood vessels, we performed immunostaining of trigeminal ganglia sections with other blood vessel markers, using anti-CD31, vascular endothelial growth factor receptor (VEGFR) 1 and VEGFR2 antibodies. In the neuronal cell body region, staining with the anti-CD31 antibody raised tubular blood vessel structures similar to those stained by the anti-CD34 antibody (Fig. 2A, C). The anti-VEGFR1 antibody reacted with neuronal cell bodies, but not with blood vessel-like tubular structures (Fig. 2E). The expression of VEGFR1 in peripheral neurons was reported in previous manuscripts, indicating possible functions of VEGFR1 in nerve remodeling in cancer and chemotherapy-induced neuropathy.29,30) No immune-reactive signal was observed with the anti-VEGFR2 antibody in the neuronal cell body region (Fig. 2G). In the fibrous region, the anti-CD31 antibody reacted with the tube-like structures but not with the line-like structures observed for the anti-CD34-antibody (Fig. 2B, D). The anti-VEGFR1-positive signals were found in linear axon-like structures, which were different from both CD31- and CD34-positive signals, suggesting VEGFR1 is expressed in peripheral neurons and localizes in the cell body and axons (Fig. 2F). VEGFR2 was not observed in the fibrous region either (Fig. 2H).
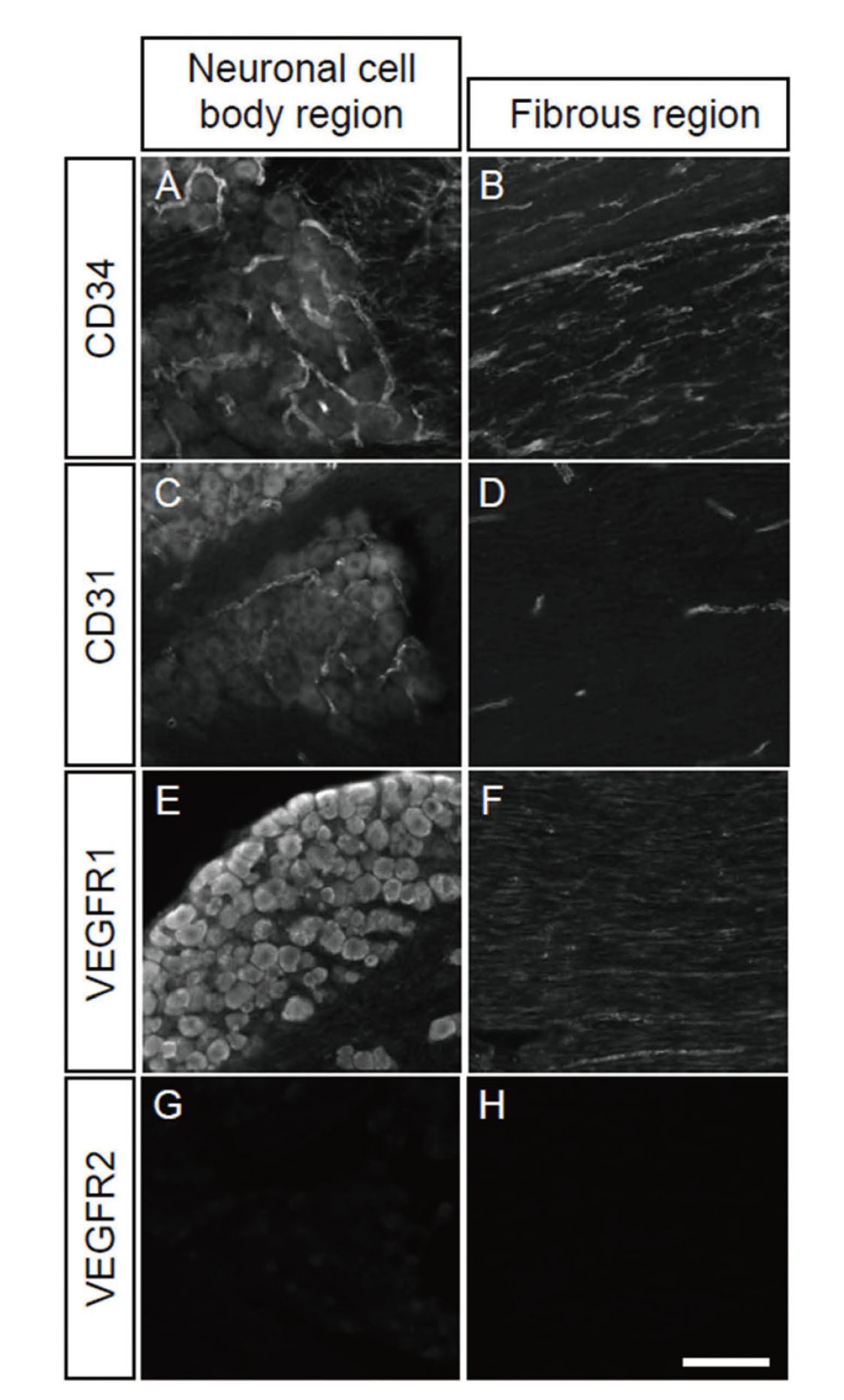
Characterization of Blood Vessels in the Trigeminal Ganglia
The trigeminal ganglia sections were stained with anti-CD34 (A, B), CD31 (C, D), VEGFR1 (E, F), or VEGFR2 (G, H) antibody. Neuronal cell body regions (A, C, E, G) and fibrous regions (B, D, F, H) were examined. Scale bar: 100 μm.
CD31, also known as platelet endothelial cell adhesion molecule-1, PECAM-1, is a cell adhesion molecule expressed in leukocyte subtypes, platelets, and vascular endothelial cells, and is widely used as a marker for endothelial cells.31) Its expression level varies among cell types and is regulated by extracellular enviroment.31) To examine whether CD31 and CD34 are detected in the same blood vessels, we performed double immunostaining with both antibodies. In the cell body region, CD31- and CD34-positive signals almost completely overlapped (Fig. 3A, C, E). Nearly all of the CD31-positive blood vessels in the fibrous region were also CD34-positive. On the other hand, only a part of CD34-positive signals was overlapped with CD31-positive signals (Fig. 3B, D, F, yellow arrows), whereas the rest of CD34-positive filaments were CD31-negative (Fig. 3F, white arrows). Thus, there are at least two different populations of blood vessels in the fibrous region.
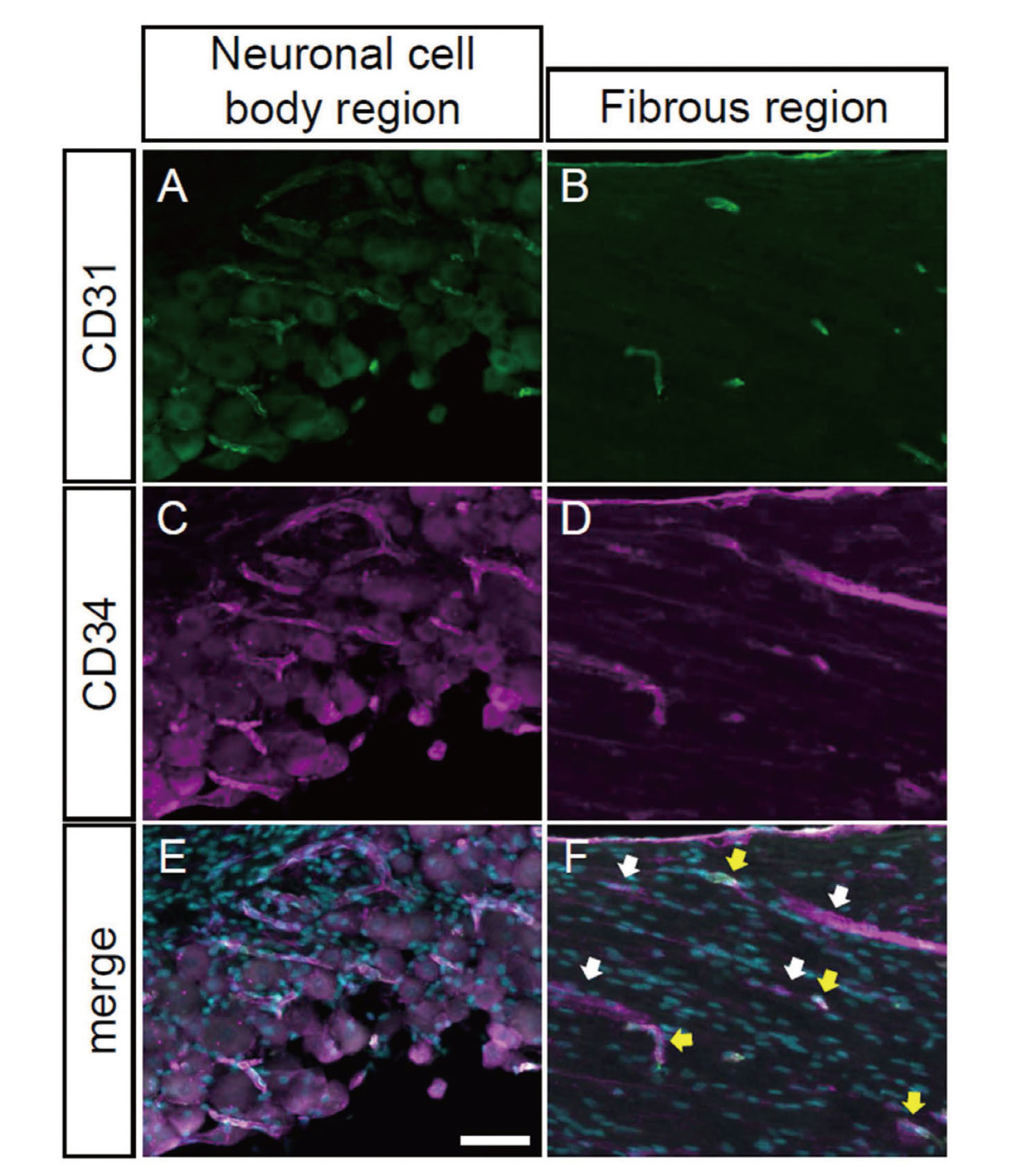
Co-expression of CD31 and CD34 in a Subpopulation of Blood Vessels
The trigeminal ganglia sections were co-immunostained with anti-CD31 (A, B) and CD34 (C, D) antibodies. Merged images with CD31- (green) and CD34- (magenta) positive signals with DAPI (cyan) were presented in panels E and F. Neuronal cell body regions (A, C, E) and fibrous regions (B, D, F) were indicated. White and yellow arrows indicate CD31- and CD34-double positive blood vessels and CD34-single positive blood vessels, respectively. Scale bar: 50 μm.
We examined the effect of repeated ethanol administration on blood vessels in the trigeminal ganglia. Ethanol was intraperitoneally injected for four consecutive days and analyzed after a one–day blank (Fig. 4A). Ethanol administration increased the number of CD34-positive blood vessels in the neuronal cell body region (Fig. 4B and C). The average length of CD34-positive vessels was shorter in the neuronal cell body region of ethanol-administered mice than that of saline-administered mice. In the fibrous region, the CD34-positive thick blood vessels presented a short and curved structure, similar to that in the neuronal cell body region, whereas those are mostly straight in the fibrous region of saline-administered mice (Fig. 4D, E). The CD34-single positive thin microvessels also underwent morphological changes, although it was not as apparent as that in thick vessels. Thus, blood vessels in the trigeminal ganglia are morphologically affected by repeated ethanol intake.
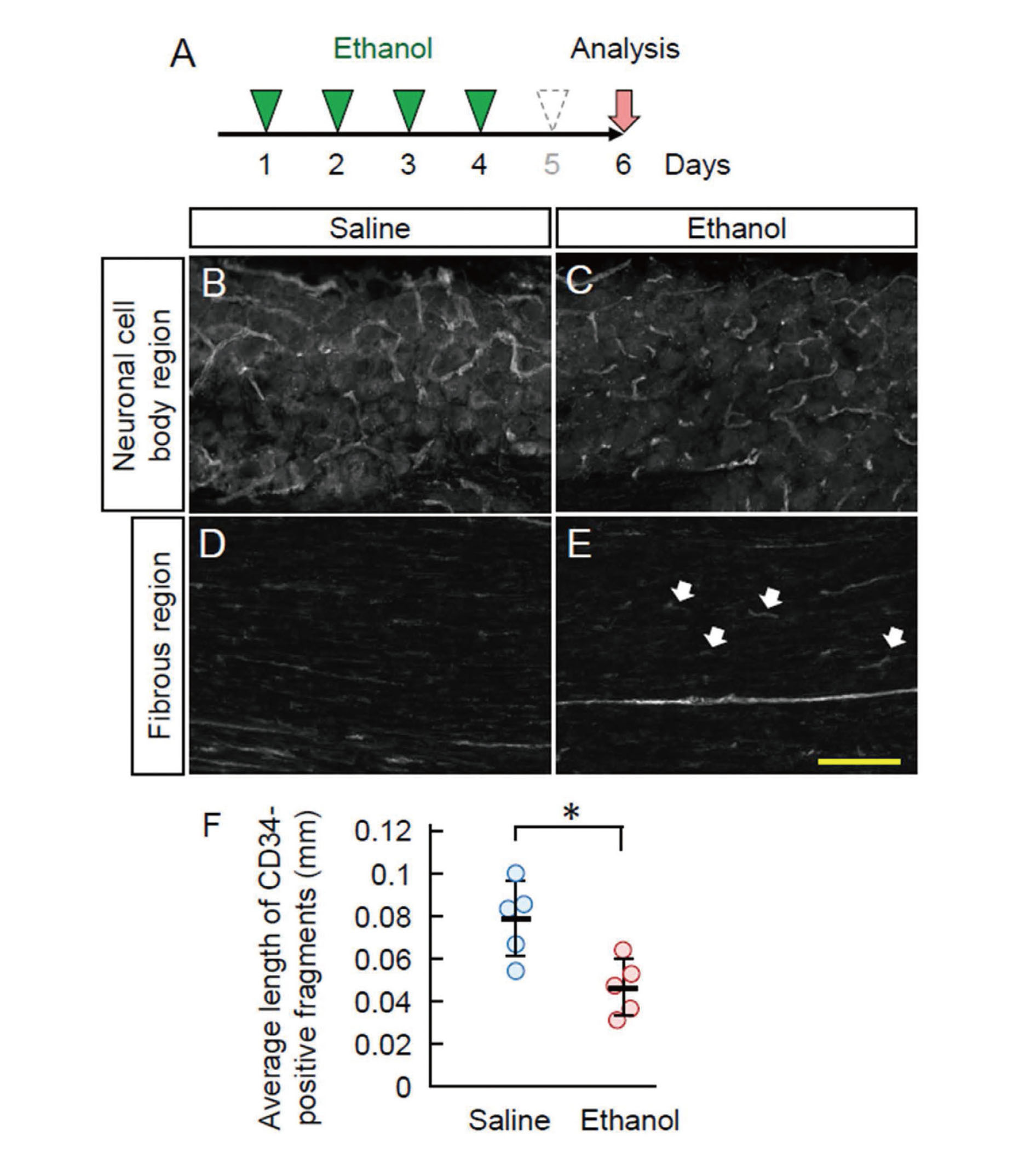
Ethanol-Induced Blood Vessel Remodeling in the Trigeminal Ganglia
A: Experimental procedure. The mice were injected with ethanol for four consecutive days and analyzed after 1 day blank. B-E: The trigeminal ganglia sections of saline- (B, D) or ethanol- (C, E) administered mice were immunostained with anti-CD34 antibody. Neuronal cell body regions (B, C) and fibrous regions (D, E) were indicated. White arrows in panel E indicate short curved CD34-positive fragments in the fibrous region. Scale bar: 100 μm. F: The average length of CD34-positive signals was quantified. Each circle indicates individual animal. Error bars indicate the standard deviation. *p < 0.05.
We next examined whether the morphological change of blood vessels is accompanied by any change in trigeminal sensory neurons. We utilized two antibodies; one of them is for a synaptic and neuron-specific protein Homer1, which is a scaffold protein associated with the plasma membrane, and the other one is for a pan-neuronal marker PGP9.5, also known as UCHL1, which mainly localizes in the cytosol.32,33) The anti-Homer1 and PGP9.5 antibodies reacted with neuronal cell bodies in the trigeminal ganglia and did not show any change in the staining pattern by ethanol administration (Fig. 5A–D). The sensory axons in the fibrous regions weakly stained by the anti-PGP9.5 antibody showed no apparent difference between saline- and ethanol-administered mice (Fig. 5E, F). Thus, repeated ethanol intake did not cause apparent morphological damage to the trigeminal neurons.

No Morphological Change in the Trigeminal Neurons
The trigeminal ganglia sections of saline- (A, C, E) or ethanol- (B, D, F) administered mice were stained with anti-Homer1 (A, B) and PGP9.5 (C-F) antibodies. The regions with both neuronal cell body and fibrous axons (A-D) and fibrous regions (E, F) were indicated. Scale bars: 50 μm.
Microglia play important roles in blood vessel homeostasis. Therefore, we examined how the microglia-like cells in the trigeminal ganglia were affected by ethanol treatment. Immunostaining with the anti-IBA1/AIF1 antibody revealed a reduction in the number of IBA1/AIF1-positive cells caused by ethanol administration (Fig. 6). Thus, repeated ethanol administration caused prolonged suppression of microglia-like cell activity. To examine the functional consequences of the suppression of microglia-like cells on blood vessels, we administered minocycline, an antibiotic with specific inhibitory activity against microglia.34-36) The administration of minocycline, instead of ethanol, imitated the morphological change of blood vessels induced by ethanol (Fig. 7A, B). The average length of CD34-positive fragments was significantly reduced by the minocycline administration (Fig. 7C).

Suppression of Microglial Activity in the Trigeminal Ganglia by Ethanol
The trigeminal ganglia sections of saline- (A, C) or ethanol- (B, D) administered mice were stained with anti-IBA1/AIF1 antibody and DAPI. Merged images with IBA1/AIF1-positive (green) and DAPI (cyan) signals were presented in panels C and D. The regions with both neuronal cell body and fibrous axons were indicated. White arrows indicate examples of IBA1/AIF1-positive active microglia. Scale bar: 50 μm.

Minocycline-Induced Blood Vessel Remodeling Similar to That by Ethanol
The trigeminal ganglia sections of saline- (A) or minocycline- (B) administered mice were stained with anti-CD34 antibody. Scale bar: 100 μm. C: The average length of CD34-positive signals was quantified. Each circle indicates individual animal. Error bars indicate the standard deviation. *p < 0.05.
In this manuscript, we examined the impact of ethanol on blood vessels within the trigeminal ganglia. Blood vessels, especially endothelial cells, have been shown to be sensitive to ethanol and exert morphological and functional defects upon ethanol exposure. Habitual ethanol consumption is associated with chronic hypertension.37) An epidemiological study suggested a positive correlation between total alcohol intake and microvascular dysfunction.38) The withdrawal of ethanol in rat subjected to repeated administration led to elevated blood pressure and increased oxidative stress.39) Ethanol exposure during developmental stages shows more prevalent long-lasting effects on the vascular system. For example, it has been linked to ectopic extra blood vessel island formation.40) Another manuscript implicated that gestational exposure to ethanol impairs the normal development of morphological and functional properties of blood vessel endothelial cells, affecting vascular network integrity, astrocyte function, and blood brain barrier maintenance in adulthood.41) Thus, the toxic effect of ethanol is associated with the long-term changes in vascular structural and functional dysfunction.7) Future studies should address the duration of ethanol-induced blood vessel remodeling observed in the trigeminal ganglia presented here to better understand its persistence. The enduring effects of ethanol extend beyond the cardiovascular system to impact various physiological systems, including the nervous and immune systems.42) Thus, blood vessel remodeling is potentially involved in broader systemic changes.7)
Previous research has suggested that ethanol induces the activation of transient receptor potential vanilloid 1 (TRPV1) and release of calcitonin gene-related peptide (CGRP) in a calcium-dependent manner.43) Activation of TRPV1 may contribute to blood vessel remodeling.44,45) CGRP is known to function in vasodilation and regulates the release of cytokines, including interleukin-1β and interleukin-6, from satellite glial cells in the trigeminal ganglia.46,47) As these inflammatory cytokines could be implicated in blood vessel dysfunction,48,49) the CGRP-controlled inflammatory cytokines might influence the ethanol-induced blood vessel remodeling in the trigeminal ganglia.
The remodeling of blood vessels may reciprocally impact neuronal and glial properties in the trigeminal ganglia. Administration of ethanol to cultured human coronary artery endothelia cells resulted in the weakened tight junction integrity and increased monocyte chemotactic protein-1 (MCP-1) production.15) Recent studies also highlighted that exposure of blood vessel endothelial cells to ethanol affects the expression of micro and long non-coding RNAs, leading to the secretion of extracellular vesicles.10,11) The physiological consequences of ethanol-induced blood vessel remodeling remain an intriguing area for future exploration.
Role of Microglia-Like Cells on the Blood Vessel Remodeling in the Trigeminal GangliaIn our results, minocycline, an inhibitor of microglial activity, induced blood vessel remodeling, similarly to that observed with ethanol (Fig. 4, 7). This underscores the crucial role of microglia-like cells in mediating ethanol-induced vascular remodeling in the trigeminal ganglia. A growing body of evidence supports the functional significance of microglia in the regulation of blood vessel across various tissues and organs. Microglia are closely associated with capillary vasculature in the central nervous system, contributing to the maintenance of blood brain barrier integrity alongside astrocytes and pericytes.50,51) Purinergic signaling-induced activation of microglia controls capillary structure, leading to capillary dilation, increased blood flow, and impaired vasodilation.52) Microglia also regulate gene expression in blood vessel endothelia cells through tissue- and cell type-specific crosstalk mediated by inflammatory cytokines.53) Despite the less understood functional roles of IBA1/AIF1-positive microglia-like cells in the trigeminal ganglia, our study suggests their involvement in the maintenance of blood vessels in the trigeminal ganglia, at least in part. Further investigations are warranted to elucidate the character and physiological roles of microglia-like cells in the trigeminal ganglia, particularly in the context of ethanol-induced neuropathies.
We thank Dr. Koji Teramoto and Ms. Tomoko Okuno, Laboratory of Hygienic Sciences, Kobe Pharmaceutical University, for their helpful support for laboratory management and technical assistance.
FundingThis work was supported by JSPS KAKENHI (JP21K07306 to HH).
Conflict of interestThe authors declare no conflict of interest.