2023 Volume 6 Issue 6 Pages 217-225
2023 Volume 6 Issue 6 Pages 217-225
Background and aim: Zinc is one of the most important essential trace elements found in more than 300 enzymes in humans, and the risk of zinc deficiency has been shown to increase with age. Symptoms associated with zinc deficiency such as muscle weakness, impaired immune function, and delayed wound healing, have much in common with health problems faced by the elderly. However, the relationship between zinc deficiency and the health problems of the elderly has not yet been fully analyzed. Here, we analyzed the effects of zinc in terms of muscle strength, skin barrier function, and gut microbiota in the elderly. Methods: Thirty elderly residents of a special nursing home in Japan were divided into two groups: a zinc intake group (14 participants) and a zinc non-intake group (16 participants). Participants in the zinc intake group received zinc (10 mg/day) for 8 weeks. Changes in muscle mass indices such as calf circumference (CC) and dry skin indices such as stratum corneum water content (SWC) were observed before and after the study. Fecal analysis was also performed at week 8 to analyze the intestinal microflora and short-chain fatty acid (SCFA) content. Results: The values of CC and SWC increased with zinc administration. The analysis of intestinal microflora showed that zinc administration increased the occupancy of Rikenellaceae. This change was observed to be associated with increased CC values. Conclusion: This study suggested that zinc may be involved in maintaining muscle mass in the elderly via the gut microbiota.
Zinc is one of the important essential trace elements contained in more than 300 enzymes in humans.1) Zinc deficiency is known to cause a variety of symptoms, including abnormal taste, muscle weakness, delayed wound healing, and immune abnormalities.2,3) Zinc deficiency in patients with cirrhosis has been shown to increase the risk of age-related sarcopenia (described below) and is also associated with frailty in the elderly;4) Yasui et al. showed that a serum zinc level below 70 μg/dL is a risk factor for severe new coronavirus infection.5) It is also becoming clear that zinc deficiency is associated with disease, as an inverse correlation was found between zinc intake and tumor frequency in a colorectal cancer induction experiment using mice.6)
High incidence of zinc deficiency in the elderly has been a growing problem in recent years. Yasuda et al. measured the zinc content in hair of Japanese subjects and reported that more than 20% of elderly subjects had zinc content below the reference value;7) Kurasawa et al. found zinc deficiency in about 20% of adults in a survey in Japan and showed that serum zinc levels decrease with age.8) A nutritional survey conducted in the United States between 1976 and 1980 also showed that serum zinc levels decrease with age and that the risk of zinc deficiency is higher in the elderly.9) Thus, zinc deficiency in the elderly is a problem not only in Japan but also in various other countries.10-14)
Elderly people suffer from various health problems, including sarcopenia due to weakened muscle strength, skin diseases due to reduced skin barrier function, and impaired immune function.15-19) Sarcopenia is one of the factors that reduce the quality of life of the elderly due to the decline in physical function, and it is known that it can be improved to some extent by diet and exercise therapy.16) It is also known that aging causes thinning of the dermis and epidermis, increased water loss, and fragmentation of collagen, leading to a decrease in barrier function,17,18) and Ikeda et al. showed that aging causes "immunosenescence," which manifests as decreased response to pathogens and vaccines and increased susceptibility to autoimmune diseases.19) Thus, the elderly face a variety of health problems, and addressing these problems is expected to improve their quality of life.
Changes in the intestinal microbiota due to aging have also received attention in recent years, and the composition of intestinal bacteria has been found to play a significant role in the health of the elderly.20-22) It has been shown that reduced diversity of the gut microbiota in the elderly compared to young adults is associated with frailty.22) Zeng et al. also showed that transplantation of fecal microbiota from young to elderly mice resulted in observed suppression of inflammation and rejuvenation of aging hematopoietic stem cells.23) Furthermore, it has been reported that zinc deficiency is associated with recurrent Clostridium difficile infection after fecal transplant treatment, suggesting that adequate intake of essential trace elements may help improve the intestinal environment.24) Therefore, control of the intestinal microbiota is very important in considering health issues in the elderly.
Zinc deficiency is one of the health problems faced by the elderly, and the aforementioned symptoms caused by zinc deficiency and the health problems faced by the elderly are thought to have much in common. However, it has not yet been fully analyzed whether the health problems of the elderly are related to the symptoms of zinc deficiency. In this study, we evaluated the changes in muscle mass, skin barrier function, and dry skin caused by zinc intake, and analyzed the intestinal microflora and short-chain fatty acid (SCFA) content in feces in order to examine the relationship between zinc deficiency and health problems in the elderly.
The study was conducted on 38 elderly residents of Sakuranomori Shiroko Home (Suzuka, Mie, Japan) from June 2019 to August 2019. Participants were randomly divided into zinc intake and zinc non-intake groups. A summary of the assessment of participant eligibility is presented in Fig. 1. Of the 38 participants, those with pressure ulcer complications (n=1), those who died en route (n=1), and those who received zinc for less than 50% of the days (n=6) were excluded from the data analysis. Ultimately, 30 participants (10 males and 20 females) completed all procedures. Of these, 14 (4 males and 10 females) received zinc and 16 (6 males and 10 females) were in a control group that did not receive zinc.

Summary of Eligibility Assessment
38 participants, those with pressure ulcer complications (n=1), those who died en route (n=1), and those who received zinc for less than 50% of the days (n=6) were excluded from the data analysis. Ultimately, 30 participants (10 men and 20 women) completed all procedures. Of these, 14 (4 males and 10 females) received zinc and 16 (6 males and 10 females) did not receive zinc.
The experimental procedure is described in Fig. 2. Prior to the study, informed consent was given to all participants. At the beginning of zinc administration, participants' transepidermal water loss (TEWL), stratum corneum water content (SWC), arm muscle area (AMA), arm circumference (AC), calf circumference (CC), triceps skinfold thickness (%TSF), and arm muscle circumference (%AMC) were measured. The zinc intake group was then given zinc (10 mg/day) for 8 weeks. The zinc supplement used was purchased from Otsuka Pharmaceutical (Tokyo, Japan). The zinc supplement used in this study contained cellulose, zinc gluconate, and sucrose fatty acid esters as ingredients. The nutritional content per tablet of the supplement was 10 mg of zinc, 1.14 kcal of energy, less than 0.1 g of protein, less than 0.1 g of fat, 0.274 g of carbohydrates, and less than 2 mg of sodium. TEWL, SWC, AMA, AC, CC, %TSF, and %AMC were measured again at 8 weeks after the start of treatment. In addition, fecal samples were collected from the participants to measure intestinal bacteria and short-chain fatty acid (SCFA) content.
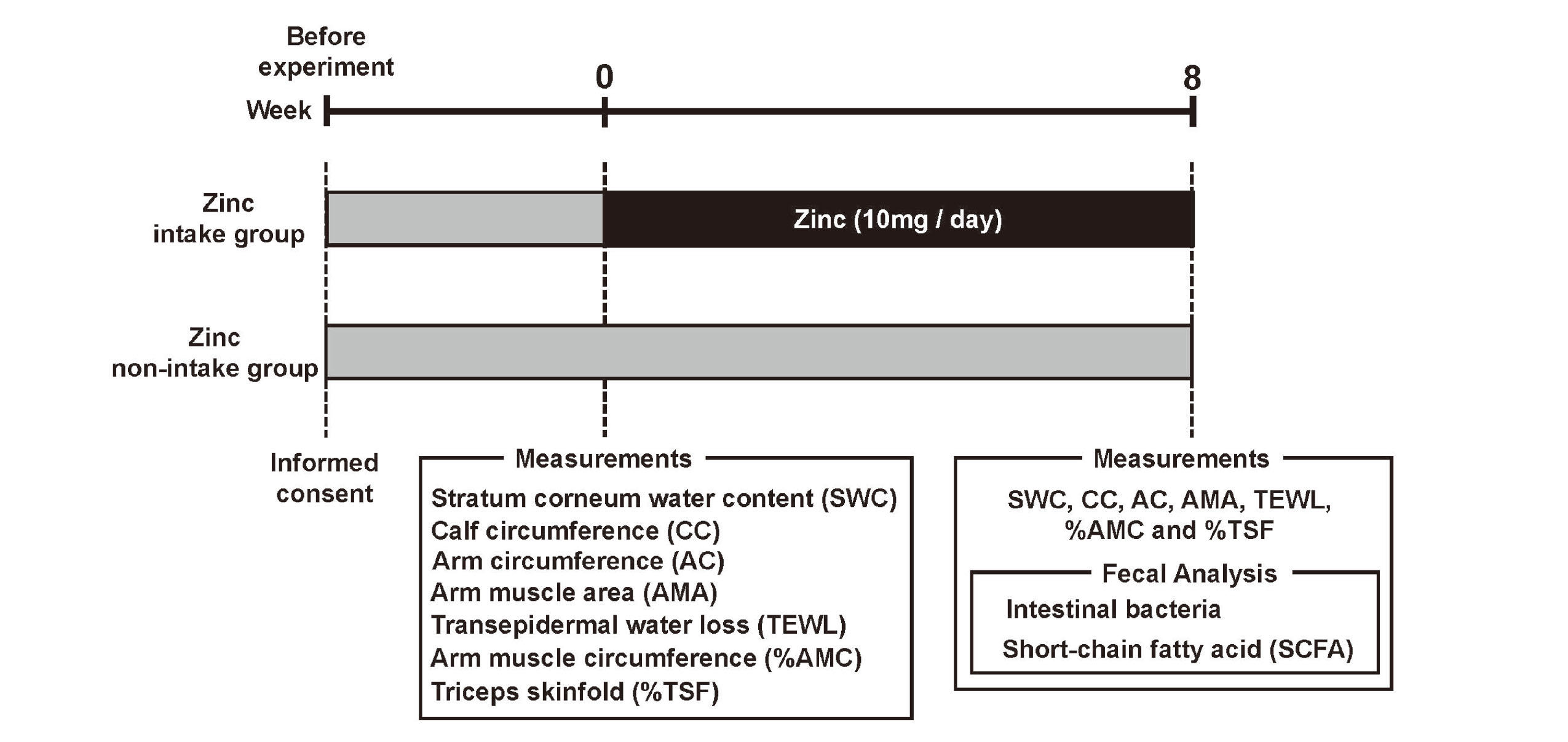
Experimental Procedure
Prior to the study, informed consent was given to all participants. At the beginning of zinc administration, participants' transepidermal water loss (TEWL), stratum corneum water content (SWC), arm muscle area (AMA), arm circumference (AC), calf circumference (CC), triceps skinfold thickness (%TSF), and arm muscle circumference (%AMC) were measured. Participants of zinc intake group then took zinc (10 mg/day) for 8 weeks. TEWL, SWC, AMA, AC, CC, %TSF, and %AMC were measured again at 8 weeks after the start of treatment. In addition, fecal samples were collected from the participants to measure intestinal bacteria and short-chain fatty acid (SCFA) content.
AMA, AC, CC, %TSF, and %AMC are known as simple and noninvasive methods to assess nutritional status, and among them, CC has been shown to be useful in assessing sarcopenia.25,26) CC was measured at the thickest part of the lower leg; AC was measured at the midpoint between the acromion and elbow head. %TSF was measured by marking the midpoint between the acromion and elbow head and pinching the skin 1 cm away from the mark so that the fat layer and muscle were separated. JARD 2001-compliant nutrition assessment kits (InserTape and Adipometer) were used to measure CC, AC, and %TSF. The %AMC and AMA were calculated from the following formula.
%AMC = AC (cm) – TSF (mm) * 3.14 / 10
AMA = %AMC^2 / (4 * 3.14)
TEWL measures the amount of water evaporation from the epidermis and is used as an indicator of the skin barrier function;27) Tewameter TM300 and Corneometer CM825 (both Courage + Khazaka electronic GmbH, Koln, Germany) were used to measure TEWL and SWC. Koln, Germany) were used for TEWL and SWC measurements. Measurements were taken three times each on the medial forearm of the non-shunt limb side, and the average of the three measurements was used for analysis. The indoor environment at the time of the measurements was 25.8 ± 1.3°C and 54.9 ± 9.3% humidity before intervention, and 25.7 ± 0.7°C and 65.1 ± 4.9% humidity after intervention.
Analysis of Intestinal MicrobiotaGut microbiota analyses were outsourced to Kyoto Institute of Nutrition & Pathology, Kyoto 610-0231, Japan. The detailed methods are available in a prior publication.28) In brief, bacterial DNA was extracted from mouse feces using a commercial extraction kit (QuickGene-810 system and QuickGene DNA tissue kit; KURABO, Osaka, Japan) as previously described.29) Microbiota composition of the feces was determined using next-generation sequencing on the MiSeq platform (Illumina, CA, USA) as previously outlined.30) PCR amplification of 16S rRNA V3-V4 region was conducted using the primers 314F and 805R. Sequencing data processing, including quality filtering, chimera checks, operational taxonomic unit definition, and taxonomy assignment, was performed using QIIME 1.9.1, USEARCH, and UCHIME, as previously detailed.31) Alpha-diversity metrics (Chao1 or Shannon index) were calculated with QIIME 1.9.1 software.
The total number of bacteria in the feces was determined through real-time PCR analysis using Roter-Gene 6200 (Qiagen). Amplification was carried out in a 10-µL reaction volume containing 5 µL of SYBR premix Ex taq (Takara Bio, Shiga, Japan), 1 µL of DNA extract, and 0.2 µmol/L of each primer. The primer sequences used were Uni331F primer, 5ʹ-TCCTACGGGAGGCAGCAGT-3ʹ, and Uni797R, 5ʹ-GGACTACCAGGGTATCTATCCTGTT-3ʹ.32) The PCR amplification protocol involved an initial denaturation step for 1 min at 95°C, followed by 35 cycles of melting, annealing, and extension at 95°C for 20 s, 63°C for 30 s, and 72°C for 45s, respectively. Total bacterial cell numbers were calculated using the Bifidobacterium longum JCM1217T cell numbers as a reference.
Organic Acid MeasurementThe measurements of concentrations of organic acids in fecal samples, including SCFA, were conducted by Kyoto Institute of Nutrition & Pathology with GCMS-QP2010 (Shimadzu, Kyoto, Japan) following a previously established protocol.33)
Statistical AnalysisTo compare two groups, the Mann-Whitney U-test was performed. For comparisons among four groups, the Kruskal-Wallis test was used. When the Kruskal-Wallis test showed a difference between the four groups, adjusted P value was calculated by Bonferroni correction following the Mann-Whitney U-test.
To calculate the odds ratios for each parameter (SWC, CC, AC, AMA, TEWL, %AMC, and %TSF) related to zinc intake, we conducted a multiple logistic regression analysis incorporating propensity scores (PS). PS were computed using the `MatchIt` package in R software (version: 4.3.1). Each participant's PS was estimated using a logistic regression model, where the dependent variable was the binary treatment assignment indicating "zinc supplement intake at 50% or more." The model's covariates included gender, age, body weight, protein intake post-intervention, and daily energy intake status post-intervention. These variables acted as confounders when estimating the PS. With the derived PS, we undertook a multivariable logistic regression analysis. In this model, our outcome variables – WCSC, CC, AC, AMA, TEWL, %AMC, and %TSF – were regressed against the PS.
The heat map was generated using the `heatmap.2` function from the `gplots` package in R software (version: 4.3.1). For the clustering process, the Euclidean distance was used as the distance metric and the complete linkage method was applied for hierarchical clustering. The vertical axis of the heat map is sorted by calf circumference change (delta CC) or stratum corneum water content change (delta SWC). From the heat map, intestinal bacteria that likely related to delta CC or delta SWC were visually selected.
Correlations between bacterial species and each parameter were tested for significance using Pearson's correlation coefficient.
For all tests, the statistical significance was set at 5%.
Limitations of ResearchThe participants in this study were older and many had poor swallowing ability, making it difficult to maintain high zinc intake rates with the tablet zinc supplement used in this study. Participants in the zinc intake group had a dietary zinc intake of approximately 6 mg per day (Table 2). Participants with a zinc intake greater than 50% of the administered zinc intake would have taken in at least an additional 5 mg of zinc per day. Participants in the zinc intake group would have a total zinc intake of at least about 11 mg per day, which is considered adequate for adult daily intake. Therefore, less than 50% zinc intake was applied as a criterion for exclusion from the data analysis in this study.
Ethical ApprovalThe study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board of Suzuka University of Medical Science (Approval No.373).
Informed ConsentWritten informed consent was obtained from all participants.
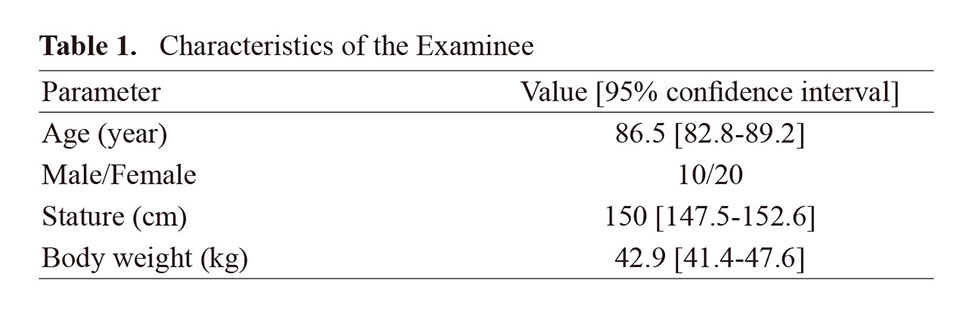
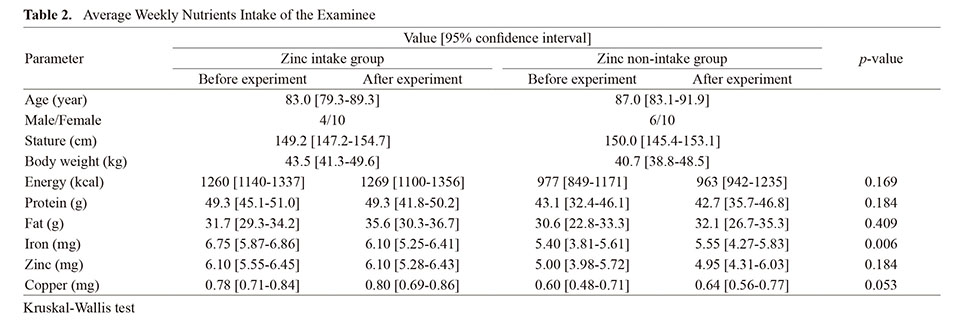
This study was conducted at a special nursing home, a Japanese public institution that accepts and provides living space for elderly people in need of care. 38 participants agreed to participate in the study. Participants with complications of pressure ulcers (n=1), participants who died during the course of the study (n=1), and participants who failed to receive zinc for more than 50% of the days (n=6) were excluded from the data analysis (Fig. 1). Finally, 30 participants provided samples for the current study and completed all procedures. The age, gender, height, and weight of the participants are shown in Table 1. Participants were divided into a zinc intake group (14 participants) and a zinc non-intake group (16 participants). There were no significant differences in dietary energy, protein, fat, zinc, or copper intake between participants in the zinc intake group and those in the zinc non-intake group (Table 2).
 Zinc Intake Improves Indices of Muscle Mass and Dry Skin in the Elderly
Zinc Intake Improves Indices of Muscle Mass and Dry Skin in the Elderly
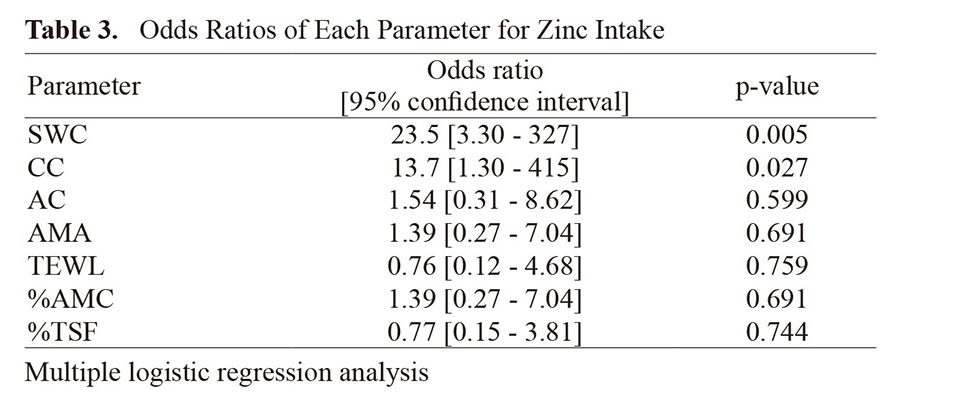
To analyze the effects of zinc intake on muscle mass and dry skin in the elderly, at weeks 0 and 8 of the intervention, participants' TEWL, SWC, AMA, AC, CC, %TSF, and %AMC were measured (Fig. 2). Multivariate logistic regression analysis showed that SWC and CC were significantly increased by zinc intake (Table 3).
 Zinc Intake Affects Gut Microbiota
Zinc Intake Affects Gut Microbiota
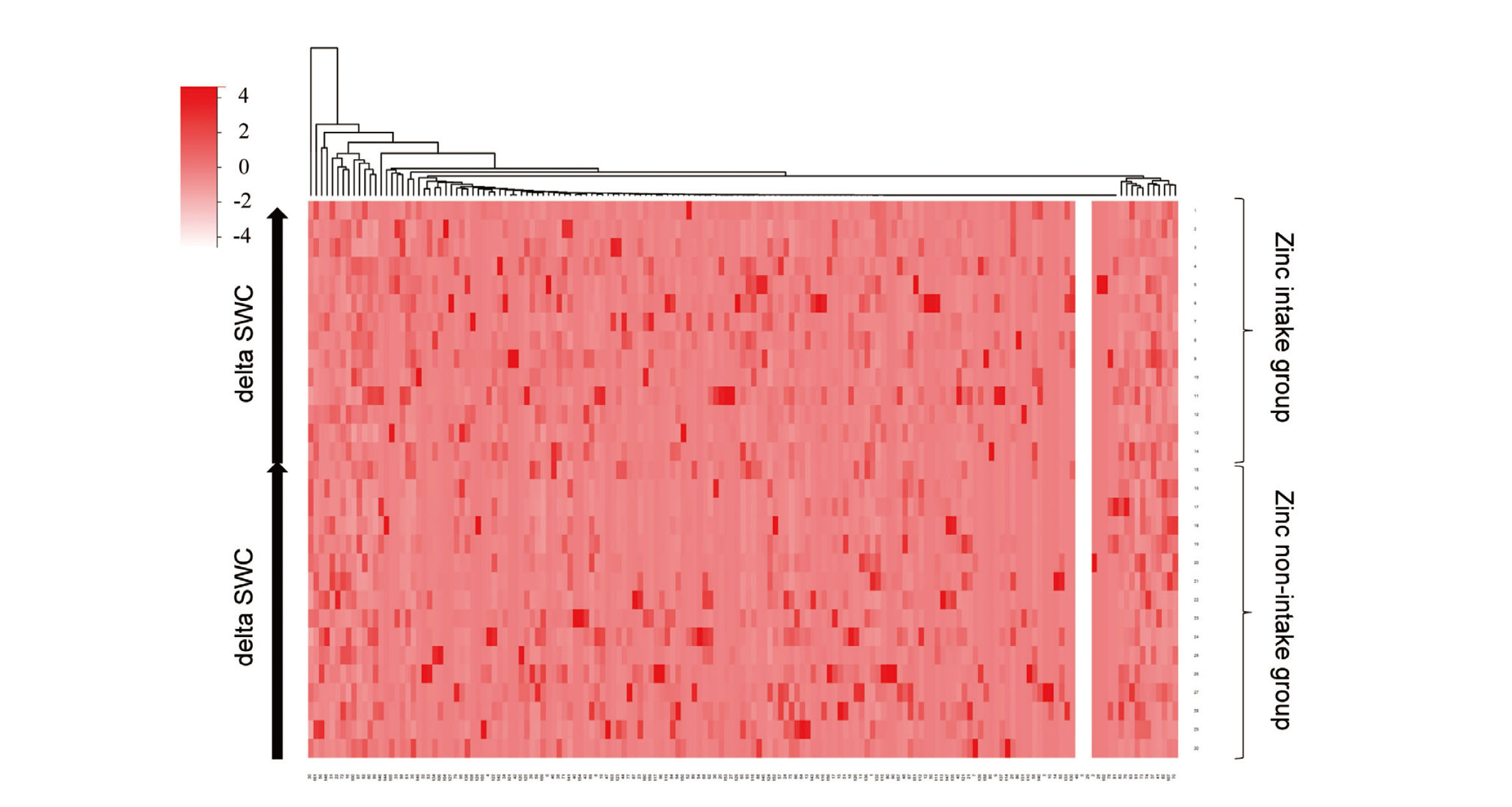
To analyze the effect of zinc on the intestinal microbiota, fecal samples from participants were collected 8 weeks after the start of zinc intake, and the intestinal microbiota and SCFA content in the feces were measured. No significant changes in estimated bacterial cell numbers in fecal samples (Fig. 3A). No significant changes in gut microbiota alpha-diversity measured by the Chao1 index and the Shannon index at the operational taxonomic unit (OTU) level (Fig. 3B, C). Bacterial species with statistically significant differences in their relative abundances are shown in Table 4.

Effects of Zinc Application on Fecal Bacterial Counts and Alpha Diversity of Gut Microbiota
(A) Estimated bacterial cell numbers in fecal samples. (B, C) Gut microbiota alpha-diversity measured by the Chao1 index (B) and the Shannon index (C) at the operational taxonomic unit (OTU) level. Zinc intake group, n = 14; Zinc non-intake group, n = 16. (bar: median with interquartile range)

The occupancies of Slackia, Rikenellaceae, a genus belonging to Clostridiales, RF39, and ML615J-28 were significantly increased in the zinc intake group, Eggerthella, Staphylococcus, Granulicatella, Pseudoramibacter_Eubacterium, Eubacterium bacteria were significantly reduced in their occupancies in the zinc intake group. The SCFA content in feces is shown in Table 5. No significant changes in SCFA content in feces were observed.
 Relationship Between Lower Leg Circumference and Rikenellaceae Occupancy
Relationship Between Lower Leg Circumference and Rikenellaceae Occupancy
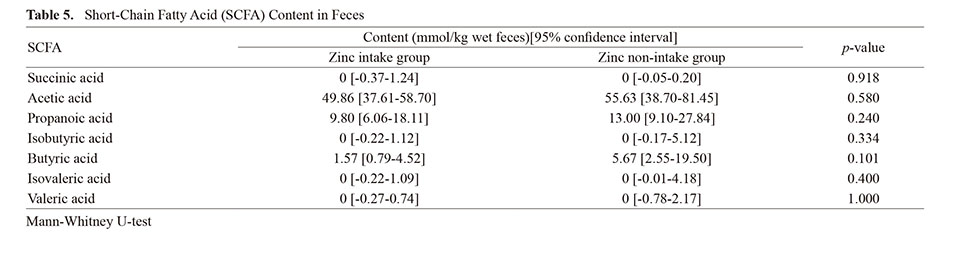
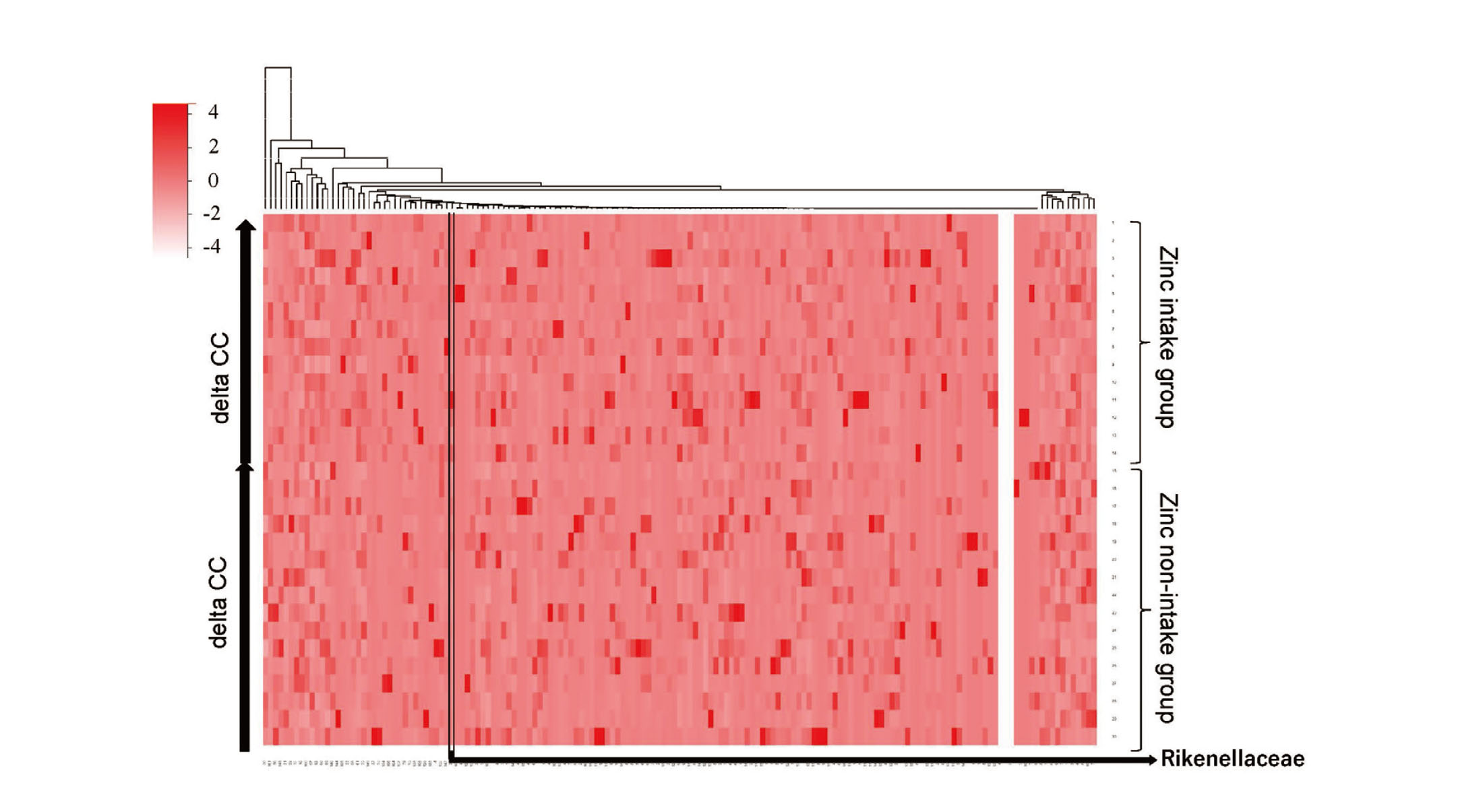
In this study, zinc intake increased the values of CC and SWC in the elderly, and changes in the intestinal microflora. Therefore, we investigated the possibility that changes in the gut microbiota due to zinc intake correlate with changes in CC and SWC. Participants were arranged in order of the amount of increase in CC, and a heat map showing the occupancy of each intestinal bacterium as indicated by color density was shown (Fig. 4). Comparing the Rikenellaceae occupancy of participants with decreased CC in the zinc non-intake group and those with increased CC in the zinc intake group, the Rikenellaceae occupancy of participants with increased CC in the zinc intake group was significantly higher (Fig. 5A). A trend was observed that increased CC was associated with increased Rikenellaceae occupancy in participants with additional zinc intake and increased CC (Fig. 5B). The intestinal bacteria associated with increased SWC could not be found in this experiment (Fig. 6).
Heat Map Showing the Relationship Between Each Intestinal Bacterium and Calf Circumference Change (Delta CC)
Participants in the zinc intake and zinc non-intake groups were arranged vertically in order of increasing delta CC, respectively, and the intestinal bacteria whose occupancy was studied were arranged horizontally. The occupancy of each intestinal bacterium is indicated by the intensity of red color. White areas indicate intestinal bacteria below the detection limit.
Relationship Between Calf Circumference Change (Delta CC) and Rikenellaceae Occupancy
(A) Rikenellaceae occupancy was compared between participants with delta CC greater than or equal to 0 and those with delta CC less than 0 in the zinc intake and zinc non-intake groups, respectively. (dot: Rikenellaceae occupancy for each participant, bar: median with interquartile range)
P value: 0.042 (for Kruskal-Wallis test)
P values were calculated using the Kruskal-Wallis test.
* Adjusted P values (Bonferroni correction following Mann-Whitney U test)
Adjusted P values were calculated using the Mann-Whitney U test with Bonferroni correction.
(B) Delta CC and Rikenellaceae occupancy for participants with delta CC greater than 0 in the zinc intake group. (dot: delta CC and Rikenellaceae occupancy for each participant, r2: Pearson's correlation coefficient, p-value: significant test for Pearson's correlation coefficient)
Heat Map Showing the Relationship Between Each Intestinal Bacterium and Stratum Corneum Water Content Change (Delta SWC).
Participants in the zinc intake and zinc non-intake groups were arranged vertically in order of increasing delta SWC, respectively, and the intestinal bacteria whose occupancy was studied were arranged horizontally. The occupancy of each intestinal bacterium is indicated by the intensity of red color. White areas indicate intestinal bacteria below the detection limit.
With the aging of population in Japan, maintaining the health of the elderly is one of the most important issues. Because many drugs including antibacterial agents and levodopa chelate zinc and inhibit zinc absorption and the risk of zinc deficiency is increased in elderly people who frequently take these drugs.34,35) Indeed, serum zinc levels have been shown to decrease with age, suggesting that there are many potential sufferers of zinc deficiency.8) In addition, some of the health problems faced by the elderly are similar to the symptoms caused by zinc deficiency. However, the relationship between the health problems of the elderly and symptoms caused by zinc deficiency has not been fully elucidated. This study suggests that zinc may be useful in maintaining muscle strength, improving dry skin, and controlling intestinal microflora in the elderly. In particular, this study is unique in that it analyzed the composition of the gut microbiota in combination with the improvement of muscle strength maintenance and dry skin with zinc intake, making the important finding that zinc may be related to muscle strength maintenance in the elderly via the gut microbiota.
In this study, multivariate logistic regression analysis of zinc intake and CC showed that zinc intake was independently and significantly associated with "change in CC greater than 0" (Table 3). CC values have been reported to be positively correlate with appendicular skeletal muscle mass and skeletal muscle index, which are sarcopenia assessment. It is a measure that can be used to assess sarcopenia.26) In addition, in a more comprehensive nutritional study including zinc, it has been reported that poor nutritional status in the elderly results in lower CC values in both men and women.36) Thus, although it was understood that adequate nutritional intake is necessary to maintain CC values in the elderly, the specific nutrients needed to maintain CC values in the elderly were unknown. In this study, zinc intake improved CC values in the elderly, indicating that among the many nutrients, zinc is one of the most effective in preventing a decline in CC values in the elderly. The result that zinc intake facilitated an increase in the value of CC suggested that zinc intake may help maintain muscle strength in the elderly. Multivariate logistic regression analysis of zinc intake and SWC showed that zinc intake was also independently and significantly associated with "change in SWC greater than 0" (Table 3). It was reported that SWC values were significantly lower in patients with pruritus compared to those without pruritus, suggesting that SWC values is considered to be one of the indicators reflecting the health status of the skin.37) The result that SWC values are more likely to increase with zinc intake suggests that zinc intake may prevent dry skin in the elderly.
The participants in this study basically consumed the same menu of meals provided at the facility, making them suitable for analysis of the intestinal microflora. As a result of analyzing the occupancy of each intestinal bacterium from the feces of the participants, the intestinal bacteria of the phyla Bacteroidetes and Firmicutes accounted for nearly 80% of the total occupancy. The Firmicutes/Bacteroidetes (F/B) ratio of the study participants was approximately 1.5, which may be a typical composition of the intestinal microbiota in the elderly.38) In the present study, Rikenellaceae occupancy was significantly increased by zinc intake. It has been reported that Alistipes shahii, a species of Rikenellaceae, is significantly decreased in patients with sarcopenia.39) It has also been shown that elevated Rikenellaceae abundance prevents fat accumulation at the expense of muscle in the elderly and is associated with healthy metabolism in the elderly.40) Although muscle mass loss in the elderly may fluctuate depending on various factors such as nutrition and physical activity, Rikenellaceae occupancy was found to be lower in participants who did not consume additional zinc and had decreased CC in this study (Fig. 5A). On the other hand, participants with additional zinc intake and increased CC had higher Rikenellaceae occupancy (Fig. 5A). Furthermore, a trend was observed that increased CC was associated with increased Rikenellaceae occupancy in participants with additional zinc intake and increased CC (Fig. 5B). However, we found that there may be a positive correlation between muscle mass and Rikenellaceae occupancy in the elderly. Thus, it is suggested that zinc is related to the maintenance of muscle strength in the elderly via the gut microbiota.
ConclusionsWe have shown that among the health problems faced by the elderly, zinc is useful in terms of maintaining muscle strength and improving dry skin, and that zinc may be involved in maintaining muscle strength in the elderly via intestinal bacteria. Adequate zinc intake may help improve the quality of life of the elderly.
We thank M. Kato for critical reading. We thank the student of the Suzuka University of Medical Science and the staff of the Sakuranomori Shiroko Home for helping experiment.
This work was supported in part by JSPS KAKENHI Grant Number JP18H05299. N. N. was supported by the Public Interest Incorporated Foundation Tsukushi Scholarship Research Fund, and the Joint Research Program of the Institute for Genetic Medicine, Hokkaido University.
Conflict of interestThe authors declare no conflict of interest.