2025 Volume 25 Pages 19-27
2025 Volume 25 Pages 19-27
Medication-induced hiccups, although uncommon, can significantly reduce patients’ quality of life. We had previously identified nicotine as a potential trigger of drug-induced hiccups. However, the mechanisms and risk factors, particularly those related to the route of administration, remain unclear. This study used the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) to investigate nicotine-induced hiccups, particularly focusing on the routes of administration and patient factors. We analyzed 14,836,467 cases reported between January 1, 2004, and March 31, 2022, which were downloaded from the FDA website. Of the 198,556 adverse event reports for nicotine, 970 involved hiccups. We performed univariate analyses on routes of administration and drug formulations to determine their influence on the occurrence of hiccups. Furthermore, we analyzed patient information related to nicotine-induced hiccups. Men were more frequently affected by nicotine-induced hiccups than women, with a higher incidence in older patients. Oral nicotine administration via gum and lozenges was more significantly associated with the occurrence of hiccups than other routes. Nicotine-induced hiccups are influenced by the administration route, particularly oral formulations, such as gum and lozenges. These findings indicate the need for further studies to elucidate the mechanisms of nicotine-induced hiccups and to develop preventive strategies.
1. Introduction
Hiccups are caused by involuntary diaphragmatic contractions and are a commonly mild and transient condition. However, in rare cases, hiccups can become persistent and significantly impair the quality of life, disrupting activities such as speaking, eating, and sleeping [1]. These contractions, along with the simultaneous closure of the glottis, are part of a reflex arc involving both the central and peripheral nervous systems that may be mediated by neurotransmitters such as γ-aminobutyric acid, dopamine, and serotonin [2].
Nicotine is an alkaloid found in tobacco that acts on nicotinic acetylcholine receptors. The primary method of nicotine intake is through cigarette smoking or nicotine replacement therapy (NRT) products, which are used to aid in tobacco cessation. In a systematic review of 120 studies that included 92 randomized clinical trials and 28 observational trials, Mills et al. [3] concluded that NRT is generally well-tolerated. However, they observed increased risks of gastrointestinal complaints, insomnia, skin irritation with transdermal formulations, oropharyngeal complaints, and hiccups with oral formulations (gum, inhaler, lozenge, spray, and sublingual tablets). We had previously identified nicotine as a potential cause of hiccups based on an analysis of adverse event reports [4]. In this previous study, data mining techniques were used to identify suspected drugs that cause hiccups and nicotine was considered important; however, a detailed analysis of nicotine alone was not conducted. In addition, the mechanisms underlying the occurrence of drug-induced hiccups, such as those triggered by nicotine, remain poorly understood. Adverse drug-induced hiccups are rare, and their frequency and mechanisms remain unclear, which make clinical research challenging. Thus, adverse drug reaction databases are valuable in identifying the factors involved in the pathogenesis of adverse drug reactions.
In this study, we used the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) to analyze the associations between nicotine-induced hiccups and various patient- and drug-related factors. Specifically, we sought to identify the routes of administration and dosage forms that most likely induce hiccups. We analyzed a large dataset of spontaneous reports to obtain a clearer understanding of nicotine-induced hiccups. Our findings could provide valuable insights for both clinical practice and further research into the mechanisms of this underreported adverse event.
2. Material and Methods
2.1 Data extraction, cleaning, and patient information
This study analyzed data obtained from the FAERS database, a comprehensive global repository of adverse drug event information. In total, 14,836,467 cases reported between January 1, 2004 and March 31, 2022, were downloaded from the FDA website [5]. Figure 1 demonstrates the method for creating the data tables. FAERS is divided into seven tables, three tables were selected, namely, DEMO (patient information), DRUG (drug information), and REAC (adverse reactions). Duplicate entries were removed, and the tables were merged using the primary ID. FAERS classifies drugs into four categories: primary suspect, secondary suspect, concomitant, and interacting drugs. For the current study, all drugs were analyzed as suspect drugs. Adverse events were categorized using preferred terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA, version 25.0) [6], with “hiccups (PT code:10020039)” as the PT of interest. Nicotine-related cases were extracted as a “nicotine table,” from which patient characteristics, such as sex, age, and weight, were analyzed. Sex was treated as a nominal variable, and categorizations other than “male” or “female” were excluded. Fisher’s exact test was used for sex-based comparisons. The t-test was used to analyze age and weight. Only data from patients aged 18–120 years and weighing ≤400 kg were included.

Figure 1. Construction of a data analysis table
The following data tables are included in FAERS: DEMO (patient information), DRUG (drug information), INDICATION (disease information), OUTCOME (outcome information), REACTION (adverse events), REPORT SOURCES (information sources), and THERAPY (treatment duration information). Of these, the DEMO, DRUG, and REAC tables were analyzed in this study. The DRUG and REAC were combined using primary ID to create an analysis table. Furthermore, reports of adverse events caused by nicotine were extracted to create a nicotine table. Additionally, the DEMO table was merged to analyze patient information.
2.2 Administration route and dosage form
In the nicotine table, univariate analysis was performed using the occurrence of hiccups as the objective variable and the route of administration and dosage form as the explanatory variables. The p-values obtained using Fisher’s exact test and the reporting odds ratio (ROR) were calculated (Table 1). Because of the lack of standardized terminology for the drug forms, similar forms were grouped together under common descriptive terms for analysis. For example, all dosage forms containing the term “gum” were categorized as “gum,” all terms containing “patch” and “transdermal” were grouped as “transdermal patch,” and all terms containing “lozenge” were combined into the “lozenge” category.
2.3 Multivariate analysis
Logistic regression analysis was performed to extract independent risk factors for nicotine-induced hiccups, with the presence or absence of hiccups as the objective variable. In addition to patient information that was significant for the univariate analysis, the route of administration and dosage form were used as explanatory variables in separate models. A pairwise method was used to evaluate the internal correlation, which was considered to exist if the square of the Spearman’s rank correlation coefficient [ρ²] exceeded 0.9.
2.4 Statistical analysis
Continuous variables were expressed as mean ± standard error of the mean. p < 0.05 was considered statistically significant. All data analyses were performed using JMP Pro 17 (SAS Institute, Cary, NC, USA).
Table 1. Cross-tabulation and formula for the ROR of hiccups
| Hiccups | Other Adverse Events | |
|---|---|---|
| Suspected drug | n11 | n12 |
| Other drugs | n21 | n22 |
ROR =
The cross-tabulation is structured using reports of suspected drugs, all other reports, reports with hiccups, and reports without hiccups (n11–22 indicates the number of cases). The reporting odds ratio (ROR) was calculated as shown.
3. Results
3.1 Data tables
The DRUG table contained 103,252,306 entries, while the REAC table contained 44,286,467 entries. After removing duplicates in each table, the combined dataset by case ID consisted of 370,103,770 entries (198,556 cases). After extracting nicotine-related data, 198,556 entries (62,118 cases) were obtained. The resulting DEMO table, which contained 14,836,467 entries of patient information, was merged to analyze patient details (Table 2). Of these, 970 reports involved hiccups (900 cases). A single case ID may be associated with multiple drug records or adverse event records, resulting in an increase in the number of entries after merging. The difference between the number of entries and the number of cases is due to duplicates and multiple reports for the same case. As a result, the number of entries can be higher than the number of cases.
The FAERS data revealed 75 different routes of administration, and the nicotine-related reports included 24 different routes of administration. For this analysis, the routes of administration were used without processing to avoid the possibility of information loss and bias resulting from integration. On the other hand, 1616 dose forms were reported in all data, and 63 forms were included in the nicotine-related reports. For example, different dosage forms of gum, such as “chewing gum,” “medicated chewing gum,” “chewable gum,” and “oral gum,” were included; however, these were considered functionally identical and unified as “gum.” Similarly, dosage forms containing “patch,” “transtherapeutic system,” and “transdermal system” were all unified as “transdermal patch,” and all dosage forms containing lozenge were unified as “lozenge.” Standardizing the notation of the dosage forms in this way increased the accuracy of subsequent comparisons of identical dosage forms.
3.2 Patient information
Table 2 presents the results of the univariate analysis of the nicotine table. After excluding the missing values for sex, age, and weight, the analysis included 57,971, 36,498, and 12,672 cases, respectively, of which 830, 640, and 141were cases of hiccups, respectively. The analysis revealed that male sex and higher age were significantly associated with the occurrence of hiccups.
Table 2. Patient information in nicotine table
| Hiccups | Non-Hiccups | p | |||||
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 407 / 423 | 20,185 / 33,326 | <0.001 | ||||
| Age (years) | 56.41 | ± | 0.59 | 51.83 | ± | 0.08 | <0.001 |
| Weight (kg) | 80.58 | ± | 2.79 | 79.63 | ± | 0.30 | 0.676 |
3.3 Administration route and dosage form
The natural logarithm of the ROR was plotted on the horizontal axis, and the negative logarithm of the p-value was plotted on the vertical axis (Fig. 2-a and 2-b). Regarding the route of administration, only nicotine administered via the buccal route, specifically the gum and lozenge formulations, was associated with hiccups.
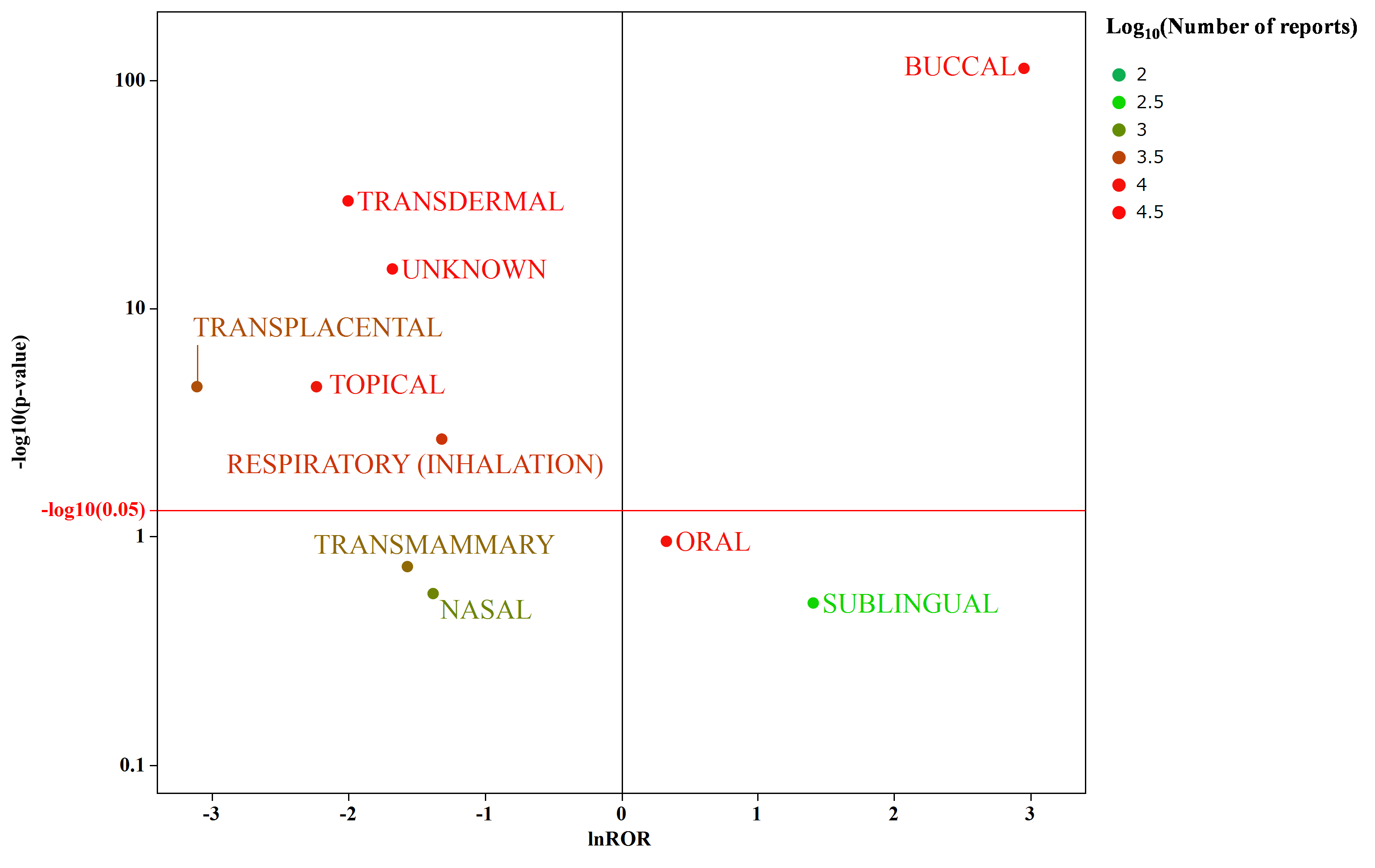
a)

b)
Figure 2. Association between the occurrence of hiccups and nicotine administration route or dosage form
Relationships between the administration route a) or dosage form b) and the occurrence of nicotine-induced hiccups. This volcano plot was constructed by plotting the negative logarithm of the p-value (−log10 p) from Fisher’s exact test on the y-axis and the natural logarithm of the ROR (lnROR) on the x-axis. The red line denotes the baseline values (p = 0.05). The colors of the individual points represent differences in the log of the number of reports for the administration route and dosage form. In this scatter plot, the signal was larger for the points (drugs) plotted in the upper right corner. The green-to-red colors indicate the number of times an adverse effect was reported.
3.4 Results of multivariate analysis
Multivariate analysis revealed that in the model including route of administration, buccal, men, and older age, which exhibited a signal in univariate analysis, were identified as independent variables. In contrast, lozenge, gum, male, and older age were identified as independent risk factors in the model including dosage form. No internal correlation was observed for each factor in each model.
Table 3. Multivariate analysis
| Route and Patients Information | Dose form and Patients Information | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Odds ratio | 95% CI | p | Factors | Odds ratio | 95% CI | p | ||||
| Buccal* | 3.20 | 2.62 | - | 3.92 | <0.0001 | Lozenge* | 5.09 | 4.17 | - | 6.21 | <0.0001 |
| Men* | 1.41 | 1.20 | - | 1.65 | <0.0001 | Gum* | 1.99 | 1.50 | - | 2.66 | 0.0005 |
| Age* | 1.02 | 1.01 | - | 1.03 | <0.0001 | Men* | 1.40 | 1.19 | - | 1.64 | <0.0001 |
| Age* | 1.018 | 1.012 | - | 1.024 | <0.0001 | ||||||
* Factors indicating independent risk
4. Discussion
This study presents the first large-scale analysis of nicotine-induced hiccups using the FAERS database. We conducted a more detailed analysis focusing on nicotine-induced hiccups, which had not been conducted previously [4]. The analysis table used in the present study contained more than 190,000 reports of nicotine-related adverse drug reactions and 900 reports of hiccups; thus, the results of this study are considered reliable. Furthermore, the route of administration and dosage form for side effects were evaluated using signal analysis. This method may be applied to the risk analysis of adverse drug reactions for other drugs in the future and is expected to contribute to the risk assessment of adverse events.
Our findings indicate that nicotine-induced hiccups are more prevalent in male and older patients, particularly involving nicotine intake via buccal routes, such as in the use of lozenges and gum. Multivariate analysis also confirmed that these factors were independent. A hiccup is an involuntary, spasmodic contraction of the diaphragm causing an initial inspiration that is suddenly checked by closure of the glottis. The glossopharyngeal nerve (ninth cranial nerve), vagus nerve (tenth cranial nerve), nuclei of the solitary tract, nucleus ambiguus, and phrenic nerve are involved in the afferent and efferent pathways of the hiccup reflex arc [1]. The administration routes and dosage forms, for which signals were detected in the present study, possibly stimulate the oral cavity or pharyngeal region, thereby triggering the glossopharyngeal nerve, which is part of the afferent pathway in the hiccup reflex arc. This hypothesis is supported by the results from a small animal study by Kondo et al. [7]. However, if nicotine stimulation in the oral and pharyngeal regions is the main cause of hiccups, then inhalation products should also increase the risk of hiccups. However, no signal was detected for inhalation products as a risk. This suggests that other mechanisms may be involved in the development of hiccups besides stimulation of the oral and pharyngeal regions.
We believe that pharmacological mechanisms must be considered as an induction pathway rather than stimulation of the oropharyngeal and pharyngeal regions by nicotine, and these mechanisms have some effect on the reflex arc of the hiccups. In addition, nicotine metabolism may be involved in the gender and age differences observed in the present study. Nicotine metabolism, influenced by CYP2A6, is more efficient in women [8], which might explain why men experience nicotine-induced hiccups more frequently. Thus, this may also explain the susceptibility of older individuals, who have slower metabolic rates. Furthermore, because hiccups are generally more common in men, the greater incidence of hiccups in men, regardless of nicotine metabolism, may have also contributed to these findings. Based on these findings, we considered both the stimulation of the oropharyngeal and pharyngeal regions by nicotine itself as well as the pharmacological effects of nicotine as possible mechanisms of nicotine-induced hiccups; however, the results of this analysis did not identify which of these is the primary factor. On the other hand, because no increased risk was observed for preparations with a relatively slow increase in blood concentration, such as transdermal preparations, we believe that a rapid increase in blood concentration may be responsible for the induction of hiccups. Although FAERS includes dosage information, there are many missing values. In particular, no cases of nicotine use and hiccups were identified; thus, an analysis could not be performed. If the relationship between nicotine dosage and hiccup onset can be established in the future, it would contribute to the estimation of pharmacological mechanisms.
Because this study included an analysis using the FAERS database, several limitations must be considered. FAERS is based on a spontaneous reporting system and is useful for detecting signals for rare and serious adverse reactions. However, it is known to have drawbacks, such as reporting bias, under-reporting of minor adverse reactions, and uncertainty in causal relationships [9]. In particular, minor side effects are less likely to be reported, whereas more serious side effects tend to be reported. Therefore, the number of reported hiccups in this study may be affected by this phenomenon. In some cases, patients themselves as well as healthcare professionals can register as reporters in FAERS. For drugs used as OTC products, such as nicotine products, the proportion of patient reports may increase, leading to higher quality and bias in reporting.
Because the signal detection method used in the present study is an imbalance analysis, “false positives” are another consideration. Therefore, in addition to ROR, the signal detection method included the number of reports and the p-value from Fisher’s exact test together to avoid a simple comparison of ROR and evaluate the results in a semiquantitative manner. The results of the disproportional analysis do not prove causality but are part of a hypothesis generation study.
Future investigations, including controlled trials or animal studies, are needed to confirm the mechanistic link between nicotine and hiccup occurrence. The results of this study provide basic information to verify the mechanism of nicotine-induced hiccups through experiments using animal models. Because no animal experiments have been previously reported that show how nicotine induces hiccups, the results obtained in this study, including the route of administration, may contribute as guidelines for future study designs. The role of nicotine metabolism and sex differences in hiccup susceptibility should also be analyzed.
5. Conclusion
This study examined the association between nicotine-induced hiccups and specific routes of administration using data obtained from the FAERS. Based on our hypothesis, it provided new insights into patient characteristics and routes of administration. Specifically, it revealed a potential association between nicotine-induced hiccups and oral formulations, such as gum and lozenges. However, our findings do not directly translate into clinical practice. To validate these results, analyses of additional patients undergoing NRT will be necessary.
Furthermore, if the risk factors and mechanisms underlying nicotine-induced hiccups are identified, it will enable healthcare providers to explain this adverse effect to patients in advance, thus alleviating their anxiety and enabling them to select appropriate medications. Additionally, understanding the mechanisms that trigger hiccups will contribute to drug development by facilitating the prediction and prevention of hiccup-related side effects.
Acknowledgements
The authors would like to acknowledge Dr. Robin Corelli for providing critical feedback and helpful suggestions to improve this manuscript.