2019 Volume 84 Issue 3 Pages 207-210
2019 Volume 84 Issue 3 Pages 207-210
The present communication reports the meiotic analysis on three wild populations of Rorippa palustris collected from Ladakh division, Jammu & Kashmir and records the existence of first ever intraspecific euploid cytotypes, the diploid (2n=14) and hexaploid (2n=42). Beside chromosome number, the two cytotypes also differ in morphometric parameters such as flower color, plant height, size of leaf and number of leaves per plant. The 6x plants grow much taller and robust compared to those of 2x and size of stomata and pollen grains are also significantly larger in the 6x compared to the 2x. Both cytotypes exhibit normal meiotic behavior characterized by normal chromosome pairing, regular segregation of chromosomes and nearly perfect pollen fertility.
Rorippa palustris (L.) Besser., (Brassicaceae) is an annual or perennial glabrous or hairy tall herb growing in marshy areas between 2000–3300 m. The species is widespread and native to parts of Africa and, much of North America and the Caribbean. To assess the cytological status of R. palustris, field surveys were conducted in the Suru Valley and Drass Valley of Ladakh division in Jammu and Kashmir. Earlier chromosomal studies in the species by Naqshi and Javeid (1976) from India and several cytologists from outside of India Jonsell (1968), Javurkova-Kratochvilova and Tomsovic (1972), Löve (1982), Zhukova (1982) and Mulligan (1984) were confined to merely counting the chromosome number and no attempt has been made to correlate the extent of morphological variability among different cytotypes and their relative distribution patterns. Looking at its wider distribution and variations in habitat, cytomorphological studies were undertaken on an individual plant basis in respect to phytogeography and cytology from cold deserts of Ladakh division. Consequent to accession based analysis, plants with two chromosome numbers, 2n=14 and 2n=42 were detected. Therefore, the present investigations were carried out with the following aims, (i) to reveal the exact chromosome number on individual plant basis (ii) to analyze the meiocytes for the meiotic course including microsporogenesis and pollen fertility (ii) to pinpoint the morphometric parameters which could be employed to segregate the variants.
Materials for male meiotic studies were collected from Suru Valley (PUN 62553) and Drass Valley (PUN 62554, 62555) within elevations of 3100–3250 min Ladakh division of J&K. Cytologically examined specimens were deposited in the Herbarium, Department of Botany, Punjabi University, Patiala (PUN).
Cytological studies and pollen fertilityFor the investigation of gametic chromosome number, meiotic course and pollen fertility, young and developing floral buds were fixed in a freshly prepared Carnoy’s fixative (6 ethanol : 3 chloroform : 1 glacial acetic acid) for 24 h subsequently transferred in 70% ethanol and stored in a refrigerator until analysis. Meiocyte preparations were made by squashing the developing anthers in 1% acetocarmine. A total of 50–100 meiocytes were examined in each case for determining the chromosome number and detailed meiotic course at diakinesis, M I, A I/T I and sporad stage. Pollen fertility was estimated through stainability tests for which anthers from fully developed and opened flowers were squashed in a glycerol–acetocarmine (1 : 1) mixture. Well-filled pollen grains with stained cytoplasm were taken as fertile while shriveled or unstained/partly stained cytoplasm was scored as sterile.
Morphometric analysisThe morphometric parameters were analyzed in the specimens examined cytologically. Trichomes and stomata were measured in the abaxial epidermal peels of mature leaves after treatment with 10% KOH for 24 h and heated at 70°C. The peels were stained Safranin solution (0.1% safranin in 60% ethanol) and mounted on a glass slide with a coverslip. Stomatal index was calculated by using the following formula: SI=S/S+E×100. Where SI stands for a stomatal index, S is the number of stomata and E is the number of epidermal cells.
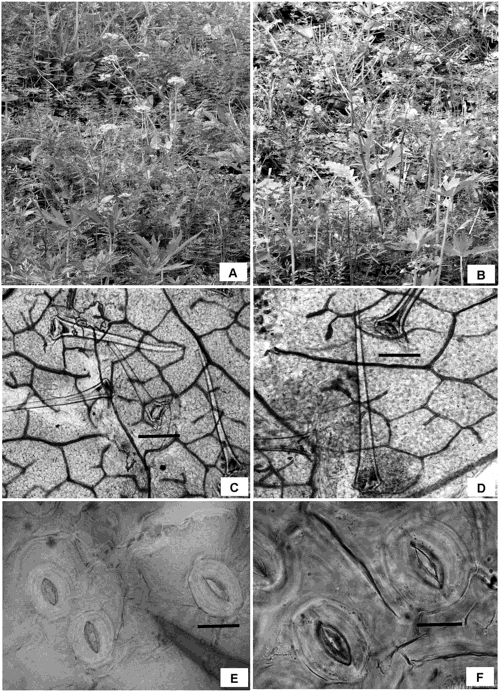
PhotomicrographsPhotomicrographs of meiotic chromosome counts, sporads, pollen grains, stomata, and trichomes were taken using a Nikon Eclipse 80i microscope.
One wild plant collected from Sankoo Village (3250 m) possessed light yellow flowers and showed a gametic chromosome number of n=7 which was ascertained from the presence of seven bivalents at diakinesis/M I (Fig. 1A, B) and 7 : 7 equal chromosomal segregation at A I (Fig. 1C). Meiotic course including sporads was normal resulting in 98% fertile pollen grains.

Two wild plants collected from Drass (3100 m) and Pandrass (3190 m) bore bright yellow flowers. These plants during meiosis showed a gametic chromosome count of n=21 as confirmed from the presence of 21 bivalents at diakinesis (Fig. 1D) and M I (Fig. 1E) and 21 : 21 chromosomes at poles during A I (Fig. 1F). The further meiotic course was perfectly regular leading to fertile pollen grains.
Morphometric analysisPlants of 2x and 6x cytotypes grow ectopically as annual herbs along roadsides and in meadows, with 2x plants noticed to be more common in the area compared to the 6x plants. Besides differing in flower color, the two cytotypes also differ in various morphometric parameters which include plant height, number of leaves/plant, size of the leaf, and flower color (Table 1). The analysis revealed that 6x plants grow much taller (90.80–105.40 cm, Fig. 2A) compared to 2x (60.50–70.40 cm, Fig. 2B). The 6x plants also possessed larger-sized leaves (18.0–26.2 cm×1.8–3.0 cm) and flowers (22.0–32.0 cm) compared to those of 2x (15.0–18.0×1.0–2.5; 20.0–25.0 cm). These cytotypes also differ at a cellular level which was reflected in the size of stomata, trichomes and pollen grains. Leaf trichomes were noticed to be larger in 6x (182.33–212.20 µm×38.22–42.40 µm) compared to the 2x (126.46–148.84 µm×30.34–34.10 µm) (Fig. 2C, D). Stomata were also recorded to be larger sized in 6x (28.02–32.78 µm×22.42–24.04 µm) compared to the 2x (23.01–26.47 µm×20.87–22.15 µm) (Fig. 2E, F). Pollen diameter was also recorded to be larger in the 6x (90.48–98.35 µm×76.96–79.38 µm, Fig. 1G) compared to the 2x (82.26–88.84 µm×66.33–70.10 µm, Fig. 1H).
| Parameters | Cytotypes | |
|---|---|---|
| Diploid (n=7) | Hexaploid (n=21) | |
| Habitat | Meadows and roadsides | Streamsides and grasslands |
| Plant height (cm) | 65.93±3.17 | 98.28±5.25 |
| No. of leaves/plant | 18–30 | 22–34 |
| Leaves | ||
| Texture | Pubescent or glabrous | Pubescent |
| Shape | Oblong | Oblong or elliptic |
| Size (cm) | 16.66±1.049×1.91±0.54 | 23.52±2.67×2.34±0.46 |
| Flower | ||
| Colour | Light yellow | Bright yellow |
| Size (mm) | 20.0–25.0 | 22.0–32.0 |
| Stomata | ||
| Size (µm) | 24.52±1.77×21.30±0.685 | 30.12±1.68×23.20±0.61 |
| Stomatal index | 10–13 | 12–14 |
| Trichome size (µm) | 137.67±6.48×32.08±1.41 | 198.05±10.39×4.59±1.40 |
| Pollen grains | ||
| Size (µm) | 85.08±2.23×68.23±1.13 | 94.73±2.76×77.63±1.09 |
| Fertility (%) | 99 | 98–100 |
The meiotic analysis in the plants of R. palustris from two different localities of cold deserts of Ladakh division J&K revealed the presence of two varied chromosome numbers, 2n=14 and 2n=52. As the genus is tribasic (x=6, 7, 8), these chromosome numbers would correspond to diploid and hexaploid levels, respectively based on x=7. Earlier, Naqshi and Javeid (1976) from India and several cytologists from outside of India (Jonsell 1968, Javurkova-Kratochvilova and Tomsovic 1972, Löve 1982, Zhukova 1982, Mulligan 1984) have recorded the tetraploid chromosome count of 2n=32 (based on x=8), suggesting the existence of dysploidy (2n=14, 16) and euploidy (2n=14, 42 and 16, 32) within the species.
Polyploidy has been suggested to be an important feature of evolution in diversification and speciation of plants (Otto and Whitton 2000, Levin 2002, Soltis et al. 2009). New polyploids arise either due to autopolyploidization or allopolyploidization. Consequences of both types of polyploidization have important evolutionary and ecological implications (Soltis et al. 2014). On the basis of chromosome pairing, allopolyploids behave like diploids and have generally been considered to be much more common than autopolyploids, mainly due to their adoptive superiority involving normal chromosome pairing, segregation, and gametic fertility. The presently recorded 6x individuals of R. palustris showed normal meiotic behavior with perfect bivalents, regular segregation, normal sporads, and high pollen fertility.
In R. palustris polyploidy seems to have drastically affected the morphological characters like plant height, size of leaves and flowers color. Such an effect of polyploidy on morphological traits including an increase in cell size of stomata, pollen grains, and trichomes is well known (Svensson 1983, Marhold 1996, Lihová et al. 2003, Španiel et al. 2008). However, there are only a few cases where polyploidy has also played a role in changing the flower color (De Schepper et al. 2001, Anssour et al. 2009, McCurthy et al. 2015).
The authors wish to thank the University Grants Commission, New Delhi (India) for providing financial assistance under the Departmental Research Support Special Assistance Programme I, II and III, the assistance for strengthening of infrastructure for science and technology programme and Junior Research Fellowship to Nissar Ahmad Khan under CSIR (Award letter no. 09/140(0173)/2018-EMR-I). The authors are also thankful to the Head Department of Botany, Punjabi University, Patiala and Sophisticated Instrumentation Centre of the University for providing necessary lab facilities during the work.