2020 Volume 85 Issue 4 Pages 263-267
2020 Volume 85 Issue 4 Pages 263-267
Chromatin remodeling represents a dynamic change of open or closed chromatin structure, which is closely related to the transcriptional regulation. The regulation by the Switch/Sucrose non-fermenting (SWI/SNF) chromatin remodeling complex is crucial for embryonic, vegetative, and reproductive development. A catalytic ATPase of the SWI/SNF complex, BRAHMA (BRM), is evolutionarily conserved among eukaryotes. We focus on the role of plant BRM in the regulation of gene expression by altering the accessibility of the target DNA. We also introduce the interacting partners required for the recruitment of BRM to the target loci.
Chromatin is a dynamic structure that contributes to high-order organization of DNA in the nucleus and condensation of DNA into chromosomes for segregation (Fujimoto and Matsunaga 2016, 2017, Hirakawa and Matsunaga 2016, Hoshino et al. 2017). Failures of chromatin dynamics are important indicators of effects by chemical treatment or gamma irradiation. For example, a cytological marker, chromatin bridge, represents incomplete segregation of sister chromatids at anaphase (Cheema et al. 2018, Dhaliwal et al. 2018, Farooq and Saggoo 2018, Gupta et al. 2018, Kaur and Gupta 2018, Saggoo and Kaur 2018, Bhat et al. 2019, Grewal and Rani 2019, Khan et al. 2019, Singh et al. 2019, Tantray et al. 2019a, b, Ergin et al. 2020. Shaikh et al. 2020). At the molecular level, chromatin structure is mainly regulated by a chromatin remodeling complex via ATP hydrolysis.
SWI/SNF chromatin remodeling complexes are large multiprotein complexes that play essential roles in transcriptional regulation through chromatin structure modifications (Clapier and Cairns 2009, Hargreaves and Crabtree 2011, Clapier et al. 2017). They alter interactions between DNA and histone octamers, using energy derived from ATP hydrolysis, to regulate DNA accessibility (Saha et al. 2006, Tang et al. 2008, Cairns 2009, Narlikar et al. 2013). Switching (SWI) and sucrose non-fermenting (SNF) were originally identified by studying mating type SWI and SNF mutants in Saccharomyces cerevisiae (Neigeborn and Carlson 1984, Stern et al. 1984, Abrams et al. 1986). SWI/SNF complexes are evolutionarily conserved among eukaryotes (Sarnowska et al. 2016). The catalytic ATPase subunit, SWI2/SNF2 ATPase, was discovered in yeast and identified as BRAHMA (BRM) in Drosophila melanogaster (Tamkun et al. 1992).
Genetic experiments have revealed the core subunits of plant SWI/SNF complexes in Arabidopsis thaliana including four SWI2/SNF2 ATPases [BRAHMA (BRM)/CHROMATIN REMODELING 2 (CHR2), SPLAYED (SYD)/CHR3, CHR12/MINUSCULE 1, and CHR23/MINISCULE2], four SWI3 proteins (SWI3A–SWI3D), two SWI/SNF-ASSOCIATED PROTEINS 73 (SWP73A and SWP73B), two ACTIN-RELATED PROTEINS (ARP4 and ARP7), and a single SNF5 subunit termed BUSHY (BSH) (Jerzmanowski 2007, Han et al. 2015, Sarnowska et al. 2016). Recently, Yu et al. (2020) identified two new core subunits of BRM-containing SWI/SNF complexes in A. thaliana, BRAHMA-interacting proteins 1 (BRIP1) and BRIP2. BRIP1 and BRIP2 are required for the maintenance of the SWI/SNF complexes to ensure their proper assembly and association with chromatin (Yu et al. 2020). Among the four SWI2/SNF2 ATPases, only BRM carries a C-terminal bromodomain, which recognizes acetylated lysine residues in histone tails (Dhalluin et al. 1999, Zeng and Zhou 2002, Zaware and Zhou 2019). Therefore, BRM is thought to be the closest homolog of yeast and animal SWI2/SNF2 ATPases (Farrona et al. 2004).
BRM is thought to play crucial roles in embryonic, vegetative, and reproductive plant development by regulating transcription of relevant target genes (Hurtado et al. 2006, Kwon et al. 2006, Tang et al. 2008, Archacki et al. 2009, Clapier and Cairns 2009, Farrona et al. 2011, Li et al. 2015). In A. thaliana, BRM mutation results in pleiotropic phenotypes, such as short and branched roots (Farrona et al. 2004, Hurtado et al. 2006), twisted and downward-curling leaves (Hurtado et al. 2006), homeotic transformations in flowers, reduced embryo fertility, hypersensitivity to abscisic acid (ABA) (Han et al. 2012), and photoperiod-independent early flowering (Farrona et al. 2004, 2007).
Although BRM interacts with thousands of genes (Li et al. 2016), SWI/SNF complexes cannot bind DNA on their own; instead, they are recruited to promoter regions through interaction with DNA-binding proteins (Kadam and Emerson 2003). This review mainly focuses on the roles of BRM and its directly interacting proteins, especially DNA-binding proteins, in the context of plant chromatin remodeling.
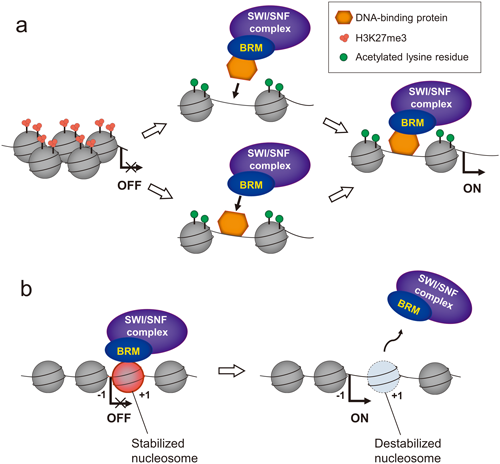
Because SWI/SNF complexes can slide nucleosomes on and eject them from many target loci but lack roles in chromatin assembly (Clapier and Cairns 2009), they were initially thought to function as transcriptional activators. In D. melanogaster, BRM was categorized as a Trithorax group (TrxG) protein because it activates homeotic gene transcription and antagonizes Polycomb group (PcG) protein function (Tamkun et al. 1992, Kal et al. 2000, Hargreaves and Crabtree 2011). As in D. melanogaster and animals, BRM in A. thaliana antagonizes PcG proteins at the target loci (Lu et al. 2011, Wu et al. 2012, Yang et al. 2015, Xu et al. 2016). PcG proteins are involved in the establishment and maintenance of the repressed chromatin state. PcG Polycomb Repressive Complex 2 (PRC2) is responsible for trimethylation of lysine 27 of histone H3 (H3K27me3), which represses gene expression at loci partially through recruitment of a second PcG complex, PRC1 (Zheng and Chen 2011). The two closely related ATPases BRM and SYD are redundantly required for activation of APETALA3 (AP3) and AGAMOUS (AG) during flower morphogenesis. Recruitment of BRM and SYD by the transcription factors LEAFY (LFY) and SEPALLATA3 (SEP3) to the regulatory regions of AP3 and AG may be necessary to overcome the Polycomb silencing of both loci (Wu et al. 2012). BRM also directly activates the expression of the key flowering time repressor SHORT VEGETATIVE PHASE (SVP) to control the flowering time in A. thaliana. In brm mutants, PcG protein occupancy and H3K27me3 levels are elevated at the SVP locus, and SVP expression is decreased (Li et al. 2015). These results indicate a crucial role for BRM in suppressing the inappropriate silencing of target genes by PcG proteins, thus maintaining appropriate expression levels of target genes during plant development (Li et al. 2015). However, it is unclear how BRM is recruited to such loci.
Li et al. (2016) identified a mechanism by which BRM is recruited to specific genomic loci: by direct physical interaction with the plant-specific H3K27 demethylase RELATIVE OF EARLY FLOWERING6 (REF6). REF6 antagonizes PcG protein activity at target loci through its zinc-finger (ZnF) domains, which directly target genomic loci containing a CTC TGYTY motif (Li et al. 2016). They showed that BRM and REF6 co-occupy many genomic loci with the CTC TGYTY motif, and REF6 is required for BRM binding to chromatin.
Recent studies in A. thaliana indicate that BRM and the histone H2A variant H2A.Z have overlapping roles that can be antagonistic or cooperative, especially in their effects on environmentally responsive genes (Torres and Deal 2019). Torres and Deal (2019) identified eight gene classes exhibiting distinct relationships with H2A.Z and BRM with respect to their roles in transcription. Moreover, BRM ChIP-seq data and MNase-seq data suggest that BRM contributes to stabilization of the nucleosome, where it binds at nucleosome-depleted regions just upstream of the +1 nucleosome. However, BRM is involved in destabilization or repositioning of flanking nucleosomes, particularly when co-localized with H2A.Z (Torres and Deal 2019). These overlapping roles of BRM and H2A.Z are also found in the regulation of the central flowering repressor FLOWERING LOCUS C (FLC). BRM represses FLC expression by creating a repressive chromatin structure at the locus where inclusion of H2A.Z would be essential for high-level expression of FLC (Deal et al. 2007, Farrona et al. 2011).
BRM can also repress target gene expression by decreasing the accessibility of the target DNA (Archacki et al. 2017). In fact, ChIP-chip experiments and transcriptional profiling of BRM showed that it activates and represses almost equal numbers of genes. Moreover, although most BRM binding sites are located at transcription start sites (TSSs), many others are located at transcription termination sites (TTSs); they have promoter-like features, such as TAT A boxes, high H3K4me3 levels, and high antisense transcriptional activity (Archacki et al. 2017). Archacki et al. (2017) speculate that TTS-bound BRM might regulate antisense transcription, which leads to the control of sense expression. In the absence of ABA, BRM promotes high occupancy and more TSS proximal positioning of the +1 nucleosome at the locus of the key transcriptional regulator of the ABA response, ABA INSENSITIVE5 (ABI5) (Han et al. 2012). When plants sense drought stress, ABA levels rise (Nambara and Marion-Poll 2005). Because nucleosomes at the TSS can act as barriers to transcription (Yen et al. 2012, Weber et al. 2014), BRM may prevent expression of ABI5 directly in the absence of drought stress. In fact, brm mutant plants exhibit ABA hypersensitivity and increased drought tolerance, similarly to the ABI5 overexpression phenotype. In addition, knocking out ABI5 activity in brm mutants partially rescues the root growth defect phenotype. These data indicate that BRM mediates the balance between growth and stress responses (Han et al. 2012).
BRM has been shown to play an important role in the regulation of major phytohormone signaling pathways, including the ABA (Han et al. 2012, Vicente et al. 2017), cytokinin (CK) (Efroni et al. 2013), auxin (Yang et al. 2015, Wu et al. 2015) and gibberellin (Archacki et al. 2013) pathways. In addition to ABA regulation, Vicente et al. (2017) showed that the C-terminus of BRM physically interacts with group VII Ethylene Response Factor (ERFVII) transcription factors in response to salt stress and ABA. ERFVIIs enhance ABI5 promoter activity by binding to a double-GCC cis-element in the ABI5 promoter that overlaps with the region occupied by BRM (Gibbs et al. 2014). It has been suggested that BRM–ERFVII interactions control plant growth through opposing functionalities, perhaps by competing for the same cis-elements (Vicente et al. 2017). In regulation of the CK pathway, BRM directly induces expression of a negative regulator of the CK response, type A ARABIDOPSIS RESPONSE REGULATOR (ARR) gene ARR16, to promote determinate leaf growth. BRM interacts with the basic-helix-loop-helix (bHLH)-related TEOSINTE BRANCHED 1, CYCLOIDEA, PROLIFERATING CELL FACTOR1/2 4 (TCP4) transcription factor; together, they bind to the region of the ARR16 promoter with TCP binding sites (GTG GTC CA and TGG TCC) (Efroni et al. 2013). In response to auxin treatment, MONOPTEROS (MP)/AUXIN RESPONSE FACTOR (ARF5) recruits BRM and SYD to increase DNA accessibility of its target genes, so that they can be switched on by transcription factors. However, in the absence of auxin, auxin-sensitive Aux/IAA proteins bind to MP/ARF5 and inhibit recruitment of BRM and SYD (Wu et al. 2015). In roots, BRM directly controls the expression of PIN-FORMED (PIN) genes in the stem cells, affecting auxin distribution. BRM mutations cause stunted root growth and defective root stem cell niche maintenance. Furthermore, in brm mutant roots, increased levels of H3K27me3 were observed in the promoters of PIN1 and PIN2, which supports the idea that BRM also antagonizes PcG function in PIN1 and PIN2 regulation (Yang et al. 2015).
It has recently been proposed that regulation of BRM stability involves the 26S proteasome and the SUMO ligase METHYL METHANE SULFONATE SENSITIVITY 21 (AtMMS21/HYP2) (Zhang et al. 2017, Sakamoto et al. 2018). BRM stability is required for tolerance of DNA double-strand breaks (DSBs), and BRM is a target of the 26S proteasome. The 26S proteasome limits the role of BRM in chromatin-loosening in association with histone acetylation to reduce susceptibility to DSB formation (Sakamoto et al. 2018). BRM directly interacts with AtMMS21, and both mutants (brm-3 and mms21-1) display similar defects in root development. Although the protein level of BRM–GFP was significantly lower in the mms21-1 mutant than in wild type, the RNA level of BRM did not change. BRM is modified by SUMO3, and AtMMS21 enhances the reaction (Zhang et al. 2017).
Several studies have suggested that SWI/SNF chromatin remodeling complexes containing BRM mediate the balance between growth and stress responses in plants. BRM can act highly selectively because it can associate with interacting factors, which controls chromatin structure and subsequently alters gene expression as described in this review (Fig. 1). Thus, further studies about how BRM activity is regulated at its target loci may enable us to understand the mechanism underlying the rapid switches in chromatin states in response to environmental and developmental signals in detail.
This research was supported by grants from MXT/JSPS KAKENHI (19H03259 and 20H03297) to SM and (19K06748 and 20H05245) to TS. We thank Candace Webb and Scott Wysong, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.