2021 Volume 86 Issue 4 Pages 311-315
2021 Volume 86 Issue 4 Pages 311-315
Heavy-ion beams have been applied as effective mutagens to various plant materials. Pollen has been used as material for mutant induction and genetic analysis. However, our knowledge of the DNA damage response of plant male gametes remains limited. In the present study, we irradiated Cyrtanthus mackenii pollen with an argon ion beam, which induced complex DNA damage, and investigated the DNA damage response of male gametes during pollen tube growth. Male gametes derived from the irradiated pollen grains were isolated from pollen tubes after 12 and 24 h of culture and subjected to cell cycle analysis. After 12 h of culture, the irradiated generative cells were frequently arrested at metaphase during pollen mitosis II (PMII), and the proportion of metaphase cells increased with increasing absorbed dose. These results suggest that the genomic lesions induced by the argon ion beam caused spindle assembly checkpoint (SAC)-dependent arrest. After 24 h of culture, the irradiated male gametes completed PMII, albeit forming sperm cells with abnormalities in chromosome separation, and chromosomal bridges were often formed between these cells. Moreover, phosphorylated H2AX foci, an indicator of DNA double-strand breaks, were detected in the irradiated male gametes after 24 h of culture, regardless of passing through the SAC. Taken together, these results indicate that male gametes activate functions to cope with radiation-induced complex DNA damage during pollen tube growth.
Heavy-ion beams produce stronger biological effects on living cells than any other radiations, such as X-rays and γ-rays, and these beams have been successfully applied as mutagens in plant breeding. Even at low doses, heavy-ion beams can induce mutations at a high frequency and induce mutants with a broad spectrum of phenotypes (Tanaka et al. 2010, Abe et al. 2015). Heavy-ion mutagenesis has been applied to various plant materials, such as dry seeds, imbibed seeds, cuttings, and tissue culture samples. As one of the materials, pollen has also been used for mutant induction and genetic analysis. For instance, in Arabidopsis thaliana, the transmissibility of deletions induced by pollen irradiation with γ-rays and carbon ion beams tends to depend on the size of the deletion; as such, while small deletions are transmitted normally, extremely large deletions are difficult to transmit to progeny (Naito et al. 2005). Silene latifolia mutants exhibiting changes in the sex phenotype have been developed using the pollen irradiation method (Kazama et al. 2016).
The effectiveness of heavy ion irradiation may be interpreted based on linear energy transfer (LET; keV·µm−1). The LET values of γ-rays and X-rays are low (0.2 and 2.0–5.0 keV·µm−1, respectively), and these rays are, therefore, called low-LET radiations. In contrast, heavy-ion beams, classified as high-LET radiation, present more variable and higher LET values than γ-rays and X-rays. Consequently, accelerated ions induce denser ionization in a more limited region than X-rays or γ-rays (Scholz 2006). Several mutation analyses in Arabidopsis have proven that the type and size of mutations induced by heavy ion beams are affected by differences in the LET values. Carbon ion beams at 22.5 and 30.0 keV·µm−1 primarily induce small mutations, such as base changes, deletions, and insertions (Kazama et al. 2011, 2017). Meanwhile, argon ion beam at 290 keV·µm−1 can induce large deletions and chromosomal rearrangements, such as inversions and translocations (Hirano et al. 2012, 2015). These results indicate that the frequency of large-scale mutations increases with increasing LET value.
In general, pollen germination is not affected even after high-dose irradiation, and seed formation and/or plant growth in the next generation are essential to determine the optimal conditions for mutation induction in pollen. Therefore, indicators of optimal conditions during pollen tube growth must be developed. In Cyrtanthus mackenii, which forms bicellular pollen, DNA damage response (DDR) during pollen tube growth following carbon ion beam irradiation has been reported (Hirano et al. 2013). Specifically, sperm cell formation was suppressed following high-dose irradiation, and cell cycle progression in the irradiated generative cells was regulated by the spindle assembly checkpoint (SAC) during pollen mitosis II (PMII). Moreover, DNA double-strand breaks (DSBs) induced in male gametes were repaired during pollen tube growth. This DDR may be one of the indicators.
In the present study, we irradiated C. mackenii pollen with an argon ion beam and explored the DDR of male gametes in detail. LET-dependent effects on male gametes were revealed by comparing the DDR between argon and carbon ion irradiation.
Anthers of C. mackenii were collected in 0.2 mL tubes and irradiated with an argon ion beam (280 keV·µm−1) at absorbed doses of 10 and 40 Gy using the E5 beam line in the RIKEN RI-beam factory. Pollen grains from the anthers were cultured in 2 mL of liquid pollen culture medium at 25°C in the dark (Hirano and Hoshino 2009).
Cell cycle phase and DNA damage analysesImmunofluorescence analysis was performed according to the method described by Hirano and Hoshino (2010), with several modifications. The generative or sperm cells in the pollen tubes were isolated after 12 or 24 h of culture and simultaneously fixed for 30 min in a fixative containing 4% (w/v) paraformaldehyde, 0.1% (v/v) glutaraldehyde, and 2% (v/v) polyoxyethylene (20) sorbitan monolaurate in the microtubule-stabilizing buffer. The cells were transferred to polylysine-coated coverslips using a microcapillary connected to a micropump (Nano Spuit; Ikeda Scientific) and then treated with a blocking agent (Image-iT FX Signal Enhancer, Thermo Fisher Scientific) for 60 min. To detect proteins using immunostaining, the cells were incubated with specific antibodies in phosphate-buffered saline supplemented with 0.05% bovine serum albumin for 60 min at 37°C. For γH2AX detection, 0.1 µg·mL−1 of anti-A. thaliana γH2AX rabbit polyclonal antibody (raised against the C-terminal peptides of Arabidopsis H2AX; KGDIGSAS(p)QEF, Sigma Genosys) was used as the primary antibody, and 5 µg·mL−1 of Alexa Fluor 488 goat anti-rabbit antibody (A11008, Thermo Fisher Scientific) was used as the secondary antibody. For microtubule staining, 1 µg·mL−1 of anti-α-tubulin mouse monoclonal antibody (A11126, Thermo Fisher Scientific) was used as the primary antibody, and 5 µg·mL−1 of Alexa Fluor 555 goat anti-mouse antibody (A21422, Thermo Fisher Scientific) was used as the secondary antibody. After immunostaining, the cells were stained with 1 µg·mL−1 of 4,6-diamidino-2-phenylindole (DAPI) or 1 µg·mL−1 of Hoechst 33258 for 15 min and mounted on coverslips in an antifade reagent (SlowFade Gold Antifade Mountant, S36937, Thermo Fisher Scientific). Images were obtained at 1.0 µm steps along the Z-axis using a confocal laser scanning microscope (LSM 700, Carl Zeiss).
In a previous study employing argon ion irradiation of C. mackenii pollen, sperm cell formation during in vitro culture was not inhibited at an absorbed dose up to 10 Gy; however, at a dose to 40 Gy, sperm cell formation in the irradiated pollens was decreased by 78% compared with that in the unirradiated controls (Hirano et al. 2018). Therefore, we focused on absorbed doses of 10 and 40 Gy and analyzed the DDR of male gametes during pollen tube growth. In C. mackenii, PMII occurs after approximately 12 h of in vitro culture (Hirano et al. 2010). We isolated male gametes from pollen tubes after 12 h of culture and determined the cell cycle phase based on the microtubule arrays (Fig. 1A). In unirradiated pollen grains, 61% of the male gametes formed sperm cells. However, the generative cells irradiated at 10 and 40 Gy were arrested at metaphase, and the proportion of metaphase cells increased with increasing absorbed dose (28% at 10 Gy and 72% at 40 Gy). Furthermore, to analyze the behavior of the arrested cells, we investigated the cell cycle phase after 24 h of culture (Fig. 1B). At this time point, all generative cells of the unirradiated controls formed pairs of sperm cells. Likewise, almost all male gametes derived from pollen grains irradiated at 10 Gy also completed PMII after 24 h of culture. However, 26% of the male gametes derived from pollen grains irradiated at 40 Gy remained at stages preceding anaphase.

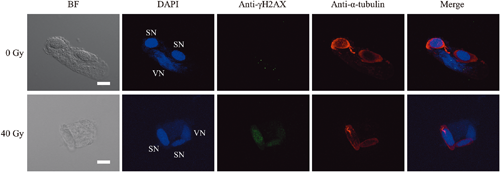
Following argon ion irradiation, abnormalities in the male gamete nuclei were observed (Fig. 2). Specifically, sperm cells with lagging chromosomes were observed more frequently in male gametes derived from pollen grains irradiated at 10 Gy (11%) than in those derived from pollen grains irradiated at 40 Gy (4%). Moreover, unequally divided sperm cells in terms of DNA content were observed. In PMII-completed cells, chromosomal bridges were formed between the sperm cells, and generative cell-like sperm cells (GC-like SCs), defined as cells with generative cell-like nuclei and sperm cell-like microtubule arrays that completed PMII but failed to separate chromosomes (Hirano et al. 2013), were also formed. Sperm cells connected by chromosomal bridges and GC-like SCs were observed after both culture periods. After 24 h of culture, respectively 32% and 33% of the male gametes derived from pollens irradiated at 10 and 40 Gy showed such connection (Fig. 1).

To reveal the repair status of DSBs, γH2AX foci, which are formed around DSBs (Lobrich et al. 2010), were detected (Fig. 3). After 12 h of culture, in pollen grains irradiated at 10 Gy, approximately 50% of the male gametes arrested at stages preceding anaphase and 30% of the male gametes arrested at anaphase and later stages contained γH2AX foci (Table 1). In pollen gains irradiated at 40 Gy, 51% of the male gametes arrested at stages preceding anaphase and 40% of the male gametes arrested at anaphase and later stages contained γH2AX foci after 12 h culture, and this proportion was respectively 59% and 47% after 24 h of culture.
| Dose (Gy) | Culture period (h) | Number of cells in each phase (%) | |||
|---|---|---|---|---|---|
| G2/prophase and metaphase | From anaphase to PMII completed cells | ||||
| with γH2AX foci | Total | with γH2AX foci | Total | ||
| 0 | 12 | 0 (0.0) | 10 | 1 (1.1) | 91 |
| 24 | 0 (0.0) | 0 | 2 (1.9) | 103 | |
| 10 | 12 | 15 (50.0) | 30 | 21 (29.6) | 71 |
| 24 | 2 (100) | 2 | 38 (33.9) | 112 | |
| 40 | 12 | 44 (51.2) | 86 | 6 (40.0) | 15 |
| 24 | 17 (58.6) | 29 | 39 (47.0) | 83 | |
In the present study, we revealed the DDR of male gametes derived from pollen grains subjected to argon ion irradiation. The irradiated generative cells were arrested at metaphase, and the increase in the proportion of metaphase cells was absorbed dose-dependent (Fig. 1). The SAC is activated by abnormal chromosomal tension due to kinetochore–spindle attachment and/or kinetochore occupancy by microtubules, and dividing cells with abnormalities are arrested at metaphase until all chromosomes achieve bipolar attachment (Musacchio and Salmon 2007). Moreover, the DDR and SAC function together at metaphase for efficient DNA repair (Eliezer 2014, Lawrence et al. 2015, Marangos et al. 2015). The interaction between DDR and SAC is thought to be conserved in Cyrtanthus male gametes, and the genomic lesions induced by argon ion irradiation likely activate DDR, leading to SAC-dependent cell cycle arrest. Cell cycle arrest at metaphase has also been observed in Cyrtanthus male gametes following carbon ion (22.5 keV·µm−1) irradiation (Hirano et al. 2013). Following carbon and argon ion irradiation, the proportion of metaphase cells after 12 h of culture was respectively 30% and 28% at 10 Gy and respectively 57% and 72% at 40 Gy. Therefore, the effect on the induction of cell cycle arrest at metaphase did not differ greatly between carbon and argon ion irradiation, suggesting that SAC-mediated arrest is activated in an absorbed dose-dependent manner, regardless of the LET value.
After PMII completion, some abnormalities derived from chromosomal rearrangements were observed in the male gamete nuclei (Fig. 3). After 24 h of culture, 27% of the male gametes derived from pollen grains subjected to 10 Gy and 62% of those derived from pollen grains subjected to 40 Gy of carbon ion irradiation formed sperm cell pairs connected by chromosomal bridges and GC-like SCs (Hirano et al. 2013), and this proportion was 32% at 10 Gy and 33% at 40 Gy of argon ion irradiation (Fig. 1). Regarding transmissible mutations in Arabidopsis (Kazama et al. 2017), argon ions (290 keV·µm−1) induced chromosomal rearrangements or large deletions more frequently than carbon ions (30 keV·µm−1). However, carbon ions produced more pronounced biological effects on chromosomal bridge induction than argon ions during the first cell division. As evidenced by the LET values, DSBs induced by carbon ions are more widely distributed in the genome, whereas those induced by argon ions are more localized. Therefore, although carbon ion irradiation may induce inter-chromosomal rearrangements leading to multi-centric chromosomes, most of these mutations would be lost during cell division and not transmitted to the next generation. Conversely, argon ion irradiation often induces transmissible rearrangements, in addition to non-transmissible ones.
The proportion of male gametes with γH2AX foci, an indicator of DSBs, tended to decrease with the prolongation of the culture period (Table 1). These data indicate that the DSBs induced by argon ions were repaired during pollen tube growth. In Cyrtanthus pollen grains irradiated with carbon ions, the male gametes passing through metaphase did not show γH2AX foci, suggesting that the generative cells with DSBs are arrested during cell cycle progression via SAC until the completion of DNA repair (Hirano et al. 2013). Interestingly, in the present study, γH2AX foci were frequently detected in irradiated male gametes passing through metaphase. High-LET radiation induces closely spaced DNA lesions, and the complexity and severity of DNA damage increase with increasing LET (Hada and Georgakilas 2008, Hagiwara et al. 2019). Clustered DNA damage is more difficult to repair (Nickoloff et al. 2020). Therefore, DSBs induced by the argon ion beam are thought to be more difficult to repair and to last longer than those induced by the carbon ion beam. Detailed molecular processes corresponding to the DDR and SAC during cell cycle progression, which may differ between male gametes with DSBs induced by the carbon and argon ion beam, must be elucidated.
Recent studies have revealed mutations induced by heavy ion irradiation at the whole-genome level, and model plants, such as Arabidopsis and rice, in the M2 and subsequent generations have been used for the analysis (Hirano et al. 2015, Kazama et al. 2017, Ichida et al. 2019). Meanwhile, experimental systems based on germ cell irradiation, such as pollen irradiation in the present study or Undaria pinnatifida sporophyll irradiation in a study by Hirano et al. (2020), can be useful to investigate the DDR during the first cell division, in addition to the transmissibility of the induced mutations and mutant screening. Exploitation of such experimental systems can further our understanding of the LET-dependent effects of radiation and offer novel insights into the DDR of germ cells.
This experiment was performed at the RIBF operated by the RIKEN Nishina Center and Center for Nuclear Study (CNS) at the University of Tokyo. This research was supported by JSPS KAKENHI Grant Numbers JP17K15223 and JP20K06035.