2021 Volume 86 Issue 4 Pages 283-289
2021 Volume 86 Issue 4 Pages 283-289
Heavy-ion beams have been widely utilized as a novel and effective mutagen for mutation breeding in diverse species, including algae, but a preferred mutant cannot be easily obtained without a suitable large-scale screening method. We devised a unique, convenient, and effective method for screening mutants of Haematococcus pluvialis to isolate a strain resistant to environmental stress with low white fluorescence, i.e., a robust strain. Haematococcus was irradiated with heavy-ion beams of carbon ions, argon ions, and iron ions at various doses, after which approximately 10,000 surviving colonies were inoculated into 96-well plates, cultured for approximately 2 weeks, and then left to dry in a refrigerator for 3–12 months without a lid. In these unattended 96-well plates, cells in approximately one-third of the wells died and became white, and the remaining wells were approximately evenly split between red and green. The robustness of wild-type and mutant strains isolated from red and green wells was compared under severe environmental-stress conditions (125 µmol photons m−2 s−1, continuous light period, 45 mM sodium acetate). In the wild-type strain, most cells died, and 93.9% of cells emitted white autofluorescence. In contrast, few G4 cells emitted white autofluorescence, indicating a survival rate of 91.8%. Strains with excellent carotenoid production, such as G7 and R1, showed greater robustness compared to wild-type strains.
Breeding with heavy-ion beam irradiation is attracting the attention of researchers (Abe et al. 2015, 2021, Kazama et al. 2018). Heavy-ion beams are characterized by high linear energy transfer (LET), and various deletion mutations can be induced in cells, from small to large, through the regulation of LET. In the case of microalgae, carotenoids are in great demand for food and cosmetic applications, and the production of strains that produce high carotenoid levels through mutation breeding techniques, such as heavy-ion beam irradiation, is more desirable than genetic modification.
Haematococcus is an egg-shaped to spherical single-celled green alga with a diameter of 20–50 µm that belongs to the order Volvocales. After endospore formation, it releases zoospores with double flagella that grow into non-motile vegetative cells. These green cells become cysts in response to environmental stressors such as strong light or low nutrition, and carotenoids such as astaxanthin that have strong antioxidant effects accumulate in the cells. The carotenoids appear to impart environmental-stress tolerance to Haematococcus (Ota et al. 2018, Ota and Kawano, 2019), but many cells cannot withstand environmental stress and become bleached, leading to death. Dead cells exhibit white autofluorescence (Takeshita et al. 2016).
We attempted to create a strain with low white fluorescence that is resistant to environmental stress; in other words, a robust mutant strain. Carotenoids produced by microalgae are used industrially as functional ingredients in cosmetics and health foods, and astaxanthin derived from Haematococcus has high marketability (Jannel et al. 2020). It is considered that microalgae strains with high environmental-stress resistance can contribute to improving mass-cultivation yields. Previous studies have demonstrated the application of heavy-ion beams to breeding Parachlorella (order Chlorellales) (Ota et al. 2013, Takeshita et al. 2018). We irradiated Haematococcus cells with heavy-ion beams, selected strains that excelled under stressful conditions, compared phenotypes, and confirmed their robustness based on white fluorescence as an indicator.
Haematococcus pluvialis strain K-0084 (H. pluvialis was recently synonymized with H. lacustris) was obtained from the Scandinavian Culture Collection of Algae and Protozoa (SCCAP) of the University of Copenhagen. Cells were precultured at 23°C under light at 10 µmol photons m−2 s−1 with a 12-h : 12-h light (L) : dark (D) cycle in a test tube (Test 30NP, Iwaki, Japan) containing 30 mL of Tris-acetate-phosphate medium (TAP, http://mcc.nies.go.jp/02medium-e.html) or BBM medium (Bischoff and Bold 1963), without agitation or aeration. One-month-old pre-culture (1–2 mL) was transferred to a conical flask containing 100 mL of TAP or BBM medium and incubated for 1 week at 23°C under light at 30 µmol photons m−2 s−1 with a 12-h : 12-h L : D cycle and agitated with a magnetic stirrer (Spinpad, Frontlab, Japan) at 50–70 rpm.
Heavy-ion beam irradiation and screeningFor mutant production using heavy-ion beam irradiation, we used the RIKEN RI Beam Factory (Wako, Saitama, Japan) with carbon ions (135 AMeV, 23 keV μm−1) at doses of 25, 50, 100, 200, and 300 Gy, argon ions (95 AMeV, 308 keV μm−1) at doses of 5, 10, 20, 40, 50, 75, and 100 Gy, and iron ions (90 AMeV, 641–816 keV μm−1) at doses of 5, 10, 20, 50, 75 and Gy. Logarithmic-phase Haematococcus cultured in BBM medium was dispensed into PCR tubes in 200-µL units or into thin plastic bags of 1–3 mL. The samples for iron-ion irradiation were placed in the plastic bags, while all other samples were placed in eight-unit PCR tubes. Samples of culture solution irradiated with heavy-ion beams of each nuclide and each dose were seeded on agar plates, and about 10,000 colonies that formed were subcultured on 96-well microplates. They were then cultured in an incubator for 3–12 months (primary screening). From the strains that survived on the primary screening plate, approximately 60 strains with high numbers of red and green cells were selected based on microscopy observations. To select carotenoid-synthesis-inducing mutants, these strains were cultured under continuous light at 125 µmol photons m−2 s−1 with 45 mM sodium acetate, and 10 strains were visually selected to represent each of the red and green traits. Analysis was performed through upscaling of this method to a 100-mL flask.
Microscopic observationsCells were observed using an Olympus BX 60 LM microscope (Olympus, Tokyo, Japan) equipped with the filter combination U-MWU2 (excitation filter 330–385 nm, absorption filter 420 nm, dichroic mirror 400 nm) to check for white fluorescence. The presence or absence of white fluorescence was confirmed through the application of 1% Evans blue stain to determine whether the cells were alive or dead. The case survival rate was calculated using the following formula.
 |
Carotenoid synthesis can be induced through the addition of sodium acetate solution (Steinbrenner and Linden 2001). A 5.7-mL volume of pre-culture grown for 2 weeks was transferred to a 12-mL culture tube (T406-2 Simport 2, BMbio, Japan), and then 300 µL of 0.9 M sodium acetate solution was added to reach a final concentration of 45 mM. The cells were cultured in an incubator (LH-40CCFL-TMDT, NK Systems, Japan) at approximately 125 µmol photons m−2 s−1 and 23°C with a 12-h : 12-h L : D cycle, and observed and measured on the 3rd and 7th days of cultivation. To measure the chlorophyll and carotenoid contents of the cells, the absorbance of pigment extracted from the cells was measured using an absorption microplate reader (Viento Nano, DS Pharma Biomedical, Japan). The chlorophyll and carotenoid contents were calculated from the measured values. The calculation formula was based on previous research (Wellburn 1994).
Haematococcus was irradiated with heavy-ion beams of carbon ions, argon ions, and iron ions at various doses (carbon ions at 0, 25, 50, 100, 200, and 300 Gy; argon ions at 0, 5, 10, 20, 40, 50, 75 and 100 Gy; and iron ions at 0, 5, 10, 20, 50 and 75 Gy). About 3,000 cells from each culture irradiated with each heavy-ion beam dose were seeded onto agar plates, and the number of colonies formed was examined for each dosage group for sensitivity testing with the heavy-ion beam (Takeshita et al. 2016). Approximately 10,000 colonies that formed on the agar plates were selected for nuclides and subcultured on 96-well plates. To isolate strains that showed high environmental tolerance, the cells were grown for approximately 2 weeks and then left to dry in the refrigerator for 3–12 months with the plate lid closed. In approximately one-third of all wells, the cells died (primary screening).
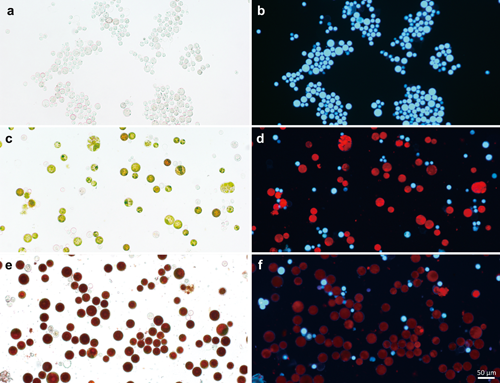
The 96-well plates that had been left unattended contained approximately equal numbers of white wells containing dead cells, green wells, and red wells (Fig. 1). When the cells in each well were observed under a microscope (Fig. 2), most of the cells in the white wells were dead, and white autofluorescence was observed (Fig. 2b). Among the cells in green wells, approximately equal numbers of green cells, white dead cells, and cells exhibiting red chlorophyll autofluorescence were observed (Fig. 2d). Most of the cells in the red wells were red, showing astaxanthin accumulation (Fig. 2e). White autofluorescence was also observed and was generally accompanied by chlorophyll red autofluorescence, but at lower intensity than in green cells. Thus, chlorophyll content apparently decreased and the levels of carotenoids such as astaxanthin increased relative to chlorophyll.

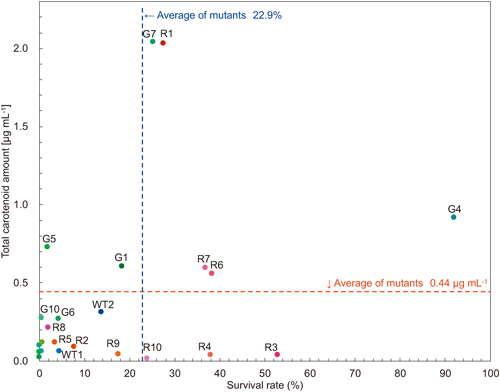
Inducing the synthesis of carotenoids such as astaxanthin in Haematococcus requires harsh conditions such as stressful light conditions or the addition of sodium acetate. Twenty strains were selected from among the strains that survived primary screening, and their carotenoid production and survival rates under the harsh conditions were plotted, together with those of three wild-type strains (Fig. 3). To induce carotenoid production, a portion of the pre-cultured culture medium was transferred to a 12-mL culture tube. After 2 weeks of culture, sodium acetate solution was added to a final concentration of 45 mM, and the mixture was incubated at 125 µmol photons m−2 s−1 for 7 days under continuous light. At that time, live and dead cells were determined through Evans blue staining and white fluorescence, and survival rates were calculated (see “Materials and methods”). The G7 and R1 strains accumulated approximately the same 2.0 µg mL−1 carotenoids per culture, significantly higher levels than the average of 0.44 µg mL−1 accumulated by other strains. Their survival rates were 25.1% (G7) and 27.3% (R1), which were slightly better than the average (22.9%). On the other hand, the survival rate of the G4 strain was as high as 91.8%, but it produced only 0.9 µg/mL of carotenoids, approximately half that of the G7 (2.0 µg mL−1) and R1 (2.0 µg mL−1) strains.
For five strains (G4, G7, R1, R6, and R7) in which both the capacity for carotenoid synthesis and the survival rate exceeded the average values, low-light conditions (30 µmol photons m−2 s−1, 12-h : 12-h L : D, designated LD) suitable for cultivation on BBM medium were used, and the cells were observed under a microscope (Fig. 4). For all strains, the cells were green, chlorophyll fluorescence was strong, and white autofluorescence emitted by dead cells was hardly observed. However, the shape of the cells differed among the strains, with R6 having many small cells, while R7 had many large cells.

To examine the robustness of the selected strains, each strain was pre-cultured in BBM medium for 2 weeks, transferred to a 12-mL culture tube, and cultured under several stress conditions. For this stress treatment, in addition to photo stress (continuous light), osmotic stress was induced through the addition of 2.5 M sorbitol solution to a final concentration of 750 mM and 0.9 M sodium acetate solution to a final concentration of 45 mM. The mortality rate of the wild-type strain was 100% when cultured in the presence of 45 mM sodium acetate for 7 days. Therefore, the wild-type strain and the mutant strain were compared in terms of robustness under severe environmental stress conditions of 125 µmol photons m−2 s−1, continuous light (LL), and 45 mM sodium acetate (Fig. 5). In the wild-type strain, most cells died, with 93.9% of cells emitting white autofluorescence. In contrast, few G4 cells emitted white autofluorescence, with a survival rate of 91.8%. Strains with excellent carotenoid production, such as G7 and R1, also showed greater robustness than the wild-type strains.

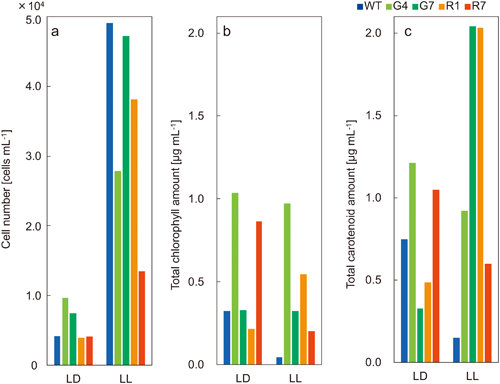
Carotenoid synthesis could not be induced by cultivation under the low-light conditions (30 µmol photons m−2 s−1, 12-h : 12-h L : D, designated LD) suitable for cultivation on BBM medium. On the other hand, carotenoid production was induced by cultivation under robust conditions, namely 125 µmol photons m−2 s−1, continuous light (LL), and 45 mM sodium acetate. However, as the numbers of dead cells increased under such harsh culture conditions, the numbers of cells and chlorophyll and carotenoid contents were measured to identify a robust strain with strong carotenoid production in the culture medium. A large difference in cell numbers was found between the LL and LD conditions, with nearly five times as many dead cells in LL as in LD. The number of wild-type cells was greatest in LL, but both chlorophyll and carotenoid contents were minimal. Although G4 was a robust strain, the number of cells (0.96×104) and chlorophyll (1.0 µg mL−1) and carotenoid contents were greatest in LD (1.2 µg mL−1). Carotenoid production was highest in G7 (2.0 µg mL−1) and R1 (2.0 µg mL−1) under the LL condition (Fig. 6).
Evans blue is commonly used to determine the life or death status of cells, as it stains dead cells blue (Baker and Mock 1994). When Haematococcus was stained with Evans blue, cells that stained blue were assumed to be dead. However, some cells that appeared to be dead were not stained, despite such cells commonly showing white fluorescence (Takeshita et al. 2016). White fluorescence will be a useful indicator of the survival of Haematococcus cells. Evans blue staining occurs through penetration of the protoplasmic membrane of destroyed protoplasts, and the thick cell wall of Haematococcus may hinder the stain’s penetration into cells. In addition, some cells stained with Evans blue but did not emit white fluorescence were observed in all cultures. These cells may be zoospores immediately after division when the cell structure is insufficiently developed, and thus may be penetrated by Evans blue stain.
As few reports are currently available on heavy-ion beam irradiation of Haematococcus (Takeshita et al. 2016), we irradiated Haematococcus with heavy-ion beams containing nuclides of carbon, argon, and iron at various doses. The survival rates were examined after treatment. Approximately 10,000 strains irradiated with heavy-ion beams were isolated and cultured under environmental-stress conditions. Microalgae for biomass production should be easy to cultivate, show resistance to environmental stress, and be amenable to mass culture. Therefore, we aimed to obtain a strain that is resistant to environmental stress and has little white fluorescence, i.e., a robust strain.
The plates that survived long-term culture for 3–12 months had a wider variety of green cells, red cyst cells, and white cells compared to plates of the wild-type strain (Fig. 1). In addition, some wells contained only cells emitting white fluorescence, while other wells contained numerous bright red and green cells with scarce white fluorescence. Therefore, a robust strain can be produced through long-term culture under harsh conditions. As long-term culture was performed in a stable low-temperature environment with constant temperature and light intensity, these differences among wells appeared in response to stressors such as oligotrophy and dryness.
A robust environment in which wild-type strains are almost eliminated is effective for inducing carotenoid synthesis (Novoveská et al. 2019, Almendinger et al. 2021). Notably, the G4 strain, which had the greatest number of surviving cells under these conditions, produced lower levels of carotenoids than did the G7 and R1 strains (Fig. 6). However, the remarkable survival rate of G4 undoubtedly lay in the antioxidant activity of carotenoids. Isolation of strains through robust screening is considered an efficient screening method, as such strains have remarkable advantages over wild-type strains and the screening method itself is very simple.
Tracing the nuclides and heavy-ion beam dosage groups of the four characteristic strains, G4, R1 and R6 were irradiated with an argon ion beam at 25 Gy, while the G7 strain was irradiated with carbon ions at 100 Gy. These strains were obtained when 3,000 cells were sown on an agar plate, forming approximately 200 colonies. Of the 20 mutant strains shown in Fig. 3, two strains were obtained by iron-ion irradiation (G9, G10), but the survival rate of each strain was 1% or less. Therefore, the iron-ion beam is not considered suitable for the production of variants through heavy-ion beam irradiation of Haematococcus, whereas 25 Gy with an argon beam and 100 Gy with a carbon ion beam were effective dosages.
This work was supported by KAKENHI Japan Science and Technology Agency, Japan; 18K19166 (Shigeyuki Kawano), and JST-OPERA Program Grant Number JPMJOP1832, Japan. The authors thank Dr. Shuhei Ota and Dr. Tomokazu Yamazaki of the University of Tokyo.