2022 Volume 87 Issue 3 Pages 285-293
2022 Volume 87 Issue 3 Pages 285-293
Coleus scutellarioides (L.) Benth. is a very popular ornamental plant with attractive colorful foliage and also has several medicinal properties. A detailed cytogenetical study was conducted in the ‘Red Trailing Queen’ horticultural form of this species. Hitherto, elaborate cytogenetic studies in this species received less attention and all of the previous studies on chromosomes are decades old. Thus, a reassessment and detailed cytogenetic study of this species are necessary. The karyotype study revealed that somatic cells contain 2n=48 small chromosomes, classified into metacentric to submetacentric. Meiotic chromosomes stained with acetocarmine and 4′,6-diamidino-2-phenylindole (DAPI) revealed the occurrence of univalent, bivalent, and multivalent associations of chromosomes along with secondary associations. The result confirms the allopolyploid origin of this plant. Meiotic irregularities like laggard chromosomes and multipolarity movement have also been found. In addition to that, variation in the chromosome numbers within pollen mother cells (PMCs) has been observed frequently. Pollen viability has been determined by fluorescein diacetate (FDA) and found to be very low. Rosmarinic acid (RA) is a natural polyphenol utilized as an anti-microbial, immunomodulatory, anti-diabetic, anti-allergic, anti-inflammatory, hepato-protectant, and renal-protectant agent. The RA content has been quantified through high-performance liquid chromatography (HPLC) to find out the medicinal efficacy. The present investigation on detailed cytogenetics study along with the pollen viability will be beneficial in the breeding program and accordingly useful in further improvement of this ornamental medicinal plant.
Coleus scutellarioides (L.) Benth. is popularly called by the synonym C. blumei Benth., is a member of the family Lamiaceae praised for its vibrant and colorful foliage (Kubínová et al. 2019). Though it is native to Java, found widely in many parts of Asia, and Europe and also cultivated in Southern Africa (Codd 1975). More than 95 synonyms present for the accepted name Coleus scutellarioides, including C. blumei, Solenostemon scutellarioides, Plectranthus scutellarioides, often create confusion (Paton et al. 2019). The same species, therefore, goes by different synonyms in different countries (Grayer et al. 2021). Moreover, several horticultural forms of Coleus are arbitrarily placed under the species C. scutellarioides. Thus, this species represents a combination or assemblage of different clones from the crosses of C. laciniatus, C. bicolor and C. atropurpureus having multi-colored variously formed leaves (Bailey 1949). The importance of Coleus in horticulture is due to its variety of ornamental foliage and trailing growth habit which is characterized by having extensive lateral branching with a reduction in apical dominance (Davies 1995). Plants of the Lamiaceae are always been a potential source for biologically active compounds and the same is true for the genus Coleus (Lukhoba et al. 2006). Besides its ornamental value, it is also used as a herbal folk medicine in many countries and simultaneously used for the large-scale production of a phenolic compound called, RA (Tungmunnithum et al. 2019). RA is an important natural product with antioxidant properties and other interesting biological activities such as anti-viral, anti-bacterial, anti-inflammatory, immunomodulatory, anti-diabetic, anti-allergic, etc. (Alagawany et al. 2017).
Exploration of chromosomes involving their number, structure, and behavior during cell division has enormous importance in understanding the cytogenetic constitution of species. Introducing beneficial traits into the plants through breeding or other biotechnological approaches also required elementary to advanced knowledge of cytogenetics. Cytological studies have been carried out in many garden forms of C. scutellarioides nearly half a century ago, hitherto elaborate analysis of mitotic and meiotic chromosomes is still inadequate (Furusato 1940, Reddy 1952, Hakeem and Rife 1966) (Table 1). Based on the multivalent association in meiotic behavior, C. scutellarioides has an allopolyploid origin (Hakeem and Rife 1966). In general, polyploidization always correspondence with extensive genomic rearrangement, gene exchange, and gene loss hence genomic instability (Adams and Wendel 2005). Such instability is also associated with chromosomal aberration from structural and numerical viewpoints that can be detected by the detailed chromosomal assessment. Sexual fitness is one of the major objects that need to be concerned before any breeding and crop improvement program. Gametes are the most precious component of sexual reproduction produced through meiosis. Consequently, analysis of chromosome behavior in meiosis is decisive in terms of investigating the sexual health of plants. Pollen is the direct product of meiosis that participates in sexual reproduction. So viable pollen after meiosis is essential for species dispersal, fitness, and the survivability of the offspring in the upcoming generation (Impe et al. 2020). Evaluation of the pollen viability thus confers pollen performance which is crucial for plant breeding and crop improvement (Dafni and Firmage 2000). During the long gap since the last chromosome assessment in this plant, a climatic shift may be taken place in India. Local adaptation of plant species after climatic shift often corresponds to the chromosomal changes and thus cytogenetic reassessment is certainly recommended. In addition to the horticultural significance, this species also contains several medicinal properties. Thus, estimation of the RA, to evaluate the medicinal efficacy of this species is needful along with the cytogenetic characterization.
| Serial | Meiosis (n) | Mitosis (2n) | Reference |
|---|---|---|---|
| 1 | 12 | Koul et al. (1976) | |
| 2 | 24 | Furusato (1940) | |
| 3 | 24, 36, 24+1 | 48+1 | Reddy (1952) |
| 4 | 24 | 48 | Hakeem and Rife (1966) |
| 5 | 24 | Mehra and Gill (1972) | |
| 6 | 24 | Bir and Saggoo (1985) | |
| 7 | 24 | Gill (1984) | |
| 8 | 30 | Singh (1985) | |
| 9 | 48 | Huziwara (1968) |
Therefore, the present investigation aimed to detail karyomorphological analysis and meiotic behavior in a horticultural form ‘Red Trailing Queen’ of C. scutellarioides. Pollen viability through the flurochromatic reaction (FCR) of FDA and seed germination rate is determined. Also, the RA content was quantified by HPLC to evaluate the medicinal efficacy of the plant. Altogether, this study will be beneficial for future biotechnological and breeding programs to improve this horticultural praiseworthy plant.
Freshly growing vegetative branches of C. scutellarioides were planted in a pot containing sand and soil in a 1 : 1 ratio and incubated in a plant growth chamber for controlling the environment (temperature 22±2°C, humidity 70%, and 16 h photoperiod). Freshly induced roots have been used for the study of mitotic cell division. The tip of growing roots was excised and pretreated with 2 mM 8-hydroxyquinoline at 16°C for 5 h. Pretreated roots were then fixed into 1 : 3 aceto–ethanol solution and kept at 4°C overnight. Roots were then hydrolyzed in 1M HCl at 60°C for 3 min, stained with 2% aceto-orcein, and squashed on a slide with 45% acetic acid. For karyomorphological studies, well-scattered metaphase plates were selected and a photomicrograph was taken by a Leica DFC295 camera. Individual chromosomes were measured with the help of the Leica application suite and categorized based on the arm ratio following Levan et al. (1964).
Meiotic studyFlower buds were fixed in 1 : 3 aceto–ethanol solution and kept at 4°C until used. Anthers were squashed on a slide and stained with 2% acetocarmine. Photomicrograph was taken by a Leica DFC295 camera. PMCs were also stained with DAPI following Santra et al. (2021) and photomicrographs were taken with an AxioCam ICc 5 and ZEN application suite equipped with a DAPI-specific filter cassette.
Pollen viability and seed germinationViable pollen grains have been detected through the FCR of the FDA following the protocol developed by Heslop-Harrison and Heslop-Harrison (1970). Stock solution 2.0 mg mL−1 FDA was prepared with acetone. Two to three anthers of the appropriate stage were squeezed in a centrifuge tube to release the pollens. Pollen was incubated for 10 min at room temperature in a 0.5 M sucrose containing 2.0 µg mL−1 FDA. Pollen viability was observed using a Zeiss Axio Scope A1 fluorescence microscope equipped with UV light and a specific filter. Photomicrographs were taken and optimized with an AxioCam ICc 5 camera and ZEN application suite
Seeds were collected from mature dry fruits, placed in a Petri dish, and maintained moisture with wet filter paper. After 7 days the seed germination percentage has been calculated.
Preparation of sample extractLeaves of C. scutellarioides were collected from the 2-years-old plants and thoroughly washed with distilled water. They were dried under 25°C under shade conditions for 7 days followed by the grind into a fine powder and stored at 4°C for future work. For the preparation of the extract, 25 mg of fine powder was dissolved with HPLC grade methanol and sonicated at 40 kHz for 30 min at 40°C. After the sonication sample was centrifuged at 6,000 rpm for 5 min and filtered the supernatant with 0.2 µm of Teflon coated filter membrane (PTFE membrane disc filters, Pall Corporation, USA) for HPLC injection.
HPLC analysis of RAThe standard of RA was purchased from Sigma-Aldrich (USA) for HPLC analysis. To prepare the stock solution, 1.0 mg mL−1 RA has been dissolved with HPLC grade methanol. Five different standard concentrations (50–500 µg mL−1) of RA were prepared from the stock solution for the calibration curve. HPLC analysis of RA was performed with binary HPLC pump (Waters 1525) and Waters 2998 PDA detector. Xbridge, C-18 reverse-phase (RP) column (5 µm particle size, 4.6×250 mm) has been used for separation of RA with two different solvent systems (Solvent A was potassium dihydrogen phosphate buffer with pH 3.2 adjusted by orthophosphoric acid and solvent B was acetonitrile) under gradient conditions (0 min 90 : 10 v/v, 13 min 78 : 22 v/v, 14 min 60 : 40 v/v, 20 min 60 : 40 v/v, 30 min 90 : 10 v/v) at 1 mL min−1 flow rate. The injection was performed with a Hamilton syringe in 20 µL of sample volume and the total run time was set for 30 min at 230 nm. The chromatographic peak of RA from sample extract was confirmed to be the retention time of standard RA. Quantification of RA was performed in Breeze 2 software.
Karyomorphological studies were carried out in the ten randomly selected somatic cells of the ‘Red Trailing Queen’ garden form of C. scutellarioides (Fig. 1A, B) and found to possess 2n=48 chromosomes in every cell (Fig. 1C, D). The chromosomes are very small in size, ranging from 1.078±0.006 to 2.536±0.045 µm. The total chromosomal length (TCL) of the haploid set is 34 µm. The primary constriction was considered as median when the arm ratio ranges from 1.05 to 1.50 and sub-median when the arm ratio ranges from 1.67 to 2.64. In the chromosome complement, the longest three pairs have submedian primary constrictions and intercalary secondary constrictions in the middle of long arms. All other 21 pairs of chromosomes have primary constrictions at the median region. The karyotype formula of the haploid set is 3sm+21 m. The detailed karyomorphological parameters are presented in Table 2 and the idiogram is constructed (Fig. 1E). According to the chromosomal asymmetry index of Stebbins (1971), the karyotype of this plant is symmetrical and is classified as 2B.
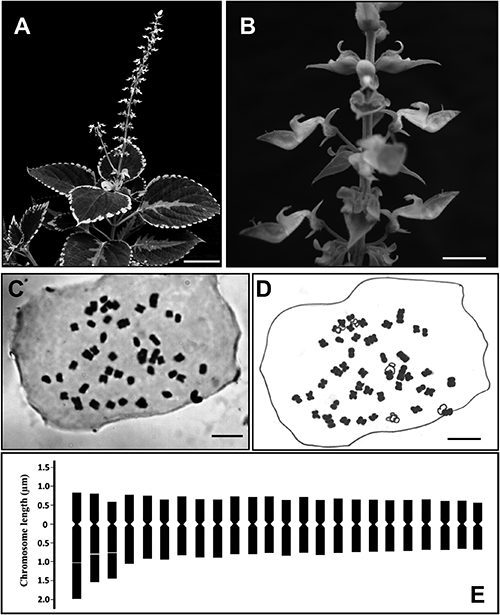
Scale bars (A)=5 cm, (B)=1 cm and (C–D)=5 µm.
| Chromosome numbers | S (µm) | L (µm) | Total length (µm) | Arm ratio (L/S) | Centromeric Index (i) | Centromeric position |
|---|---|---|---|---|---|---|
| 1 | 0.760±0.006 | 1.776±0.045 | 2.536±0.045 | 2.34 | 29.968 | sm |
| 2 | 0.731±0.011 | 1.445±0.005 | 2.176±0.015 | 1.98 | 33.594 | sm |
| 3 | 0.505±0.003 | 1.358±0.011 | 1.863±0.010 | 2.69 | 27.107 | sm |
| 4 | 0.696±0.011 | 0.977±0.043 | 1.673±0.049 | 1.40 | 41.602 | m |
| 5 | 0.671±0.003 | 0.847±0.006 | 1.518±0.009 | 1.26 | 44.203 | m |
| 6 | 0.564±0.006 | 0.873±0.004 | 1.437±0.005 | 1.55 | 39.248 | m |
| 7 | 0.665±0.005 | 0.743±0.007 | 1.408±0.007 | 1.12 | 47.230 | m |
| 8 | 0.586±0.005 | 0.803±0.015 | 1.389±0.016 | 1.37 | 42.189 | m |
| 9 | 0.570±0.007 | 0.814±0.004 | 1.384±0.006 | 1.43 | 41.185 | m |
| 10 | 0.648±0.003 | 0.727±0.010 | 1.375±0.012 | 1.12 | 47.127 | m |
| 11 | 0.628±0.008 | 0.731±0.005 | 1.359±0.006 | 1.16 | 46.210 | m |
| 12 | 0.648±0.007 | 0.691±0.006 | 1.339±0.007 | 1.07 | 48.394 | m |
| 13 | 0.562±0.015 | 0.753±0.008 | 1.315±0.014 | 1.34 | 42.738 | m |
| 14 | 0.642±0.012 | 0.670±0.006 | 1.312±0.012 | 1.04 | 48.933 | m |
| 15 | 0.560±0.013 | 0.734±0.008 | 1.294±0.019 | 1.31 | 43.277 | m |
| 16 | 0.588±0.021 | 0.695±0.008 | 1.283±0.028 | 1.18 | 45.830 | m |
| 17 | 0.568±0.007 | 0.663±0.012 | 1.231±0.010 | 1.17 | 46.141 | m |
| 18 | 0.565±0.001 | 0.649±0.011 | 1.214±0.011 | 1.15 | 46.540 | m |
| 19 | 0.547±0.007 | 0.653±0.008 | 1.20±0.015 | 1.19 | 45.583 | m |
| 20 | 0.552±0.007 | 0.635±0.006 | 1.187±0.007 | 1.15 | 46.504 | m |
| 21 | 0.568±0.008 | 0.604±0.009 | 1.172±0.009 | 1.06 | 48.464 | m |
| 22 | 0.527±0.006 | 0.614±0.011 | 1.141±0.016 | 1.17 | 46.188 | m |
| 23 | 0.530±0.007 | 0.580±0.005 | 1.11±0.011 | 1.09 | 47.748 | m |
| 24 | 0.477±0.007 | 0.601±0.009 | 1.078±0.006 | 1.26 | 44.249 | m |
S=short arm, L=long arm, m=median region, sm=submedian region.
Meiosis in the PMCs was detected with DAPI (Fig. 2A–L) as well as acetocarmine (Fig. 3A–M). Chromosomes are involved in the secondary associations in metaphase-I in addition to the primary associations that were revealed more prominently with DAPI over conventional acetocarmine stain (Fig. 2A–C). In most of the PMCs, chromosomes were involved in univalent, bivalent, trivalent, and tetravalent associations (Fig. 3A–C). In addition to the different valencies, secondary associations have also been observed (Fig. 2C). These phenomena made the scenario difficult to count the exact number of chromosomes present in the PMC. Though some of the PMCs contain n=24 chromosomes that are considered normal (Fig. 3A). The multivalent formation remain exists even after the separation in anaphase-I (Fig. 3D–F). After separation of homologous chromosomes in anaphase-I, 24 chromosomes counted in a single-pole of normal PMCs again confirm the haploid chromosome number n=24 (Figs. 2D, 3D). Among different meiotic irregularities, laggard chromosomes have been found in a large number of PMCs (86.75%). They observed in different stages like metaphase-I (Fig. 2E–F), anaphase-I (Fig. 2G), late telophase-I (Fig. 2H), after the completion of meiosis-I (Fig. 2I), and meiosis-II (Fig. 2J). The number of laggard chromosomes is also variable from one to several. In a few PMCs, unequal separation has been recorded, though the number of such PMCs is not significant (Fig. 3G). Another principal abnormality found during meiosis is the numerical variation of chromosomes in different PMCs also called chromosome mosaicism (Figs. 2K, 3H–L). A large number of PMCs (more than 500 cells) were detected having the numerical variation of chromosomes from four (Fig. 3H) to several (Fig. 3I–L). Because of the secondary associations and different chromosomal valency, counting exact chromosome numbers in every PMC was not possible. Thus, the chromosome number range and the percentage of PMCs have been represented in Table 3. Multipolarity has been detected in a few PMCs (Figs. 2L, 3M). The viable pollens develop a bright green color after FCR whereas nonviable pollens have uniform background fluorescence or ghost fluorescence (Fig. 3N). The estimated pollen viability after the evaluation of more than 2,000 pollens was 35.43% and considered very poor. Several tetrads were analyzed where only one or two cells were found to be viable out of four cells (Fig. 3O). The frequency of seed germination is good and estimated to be 87.18% (Fig. 3P)
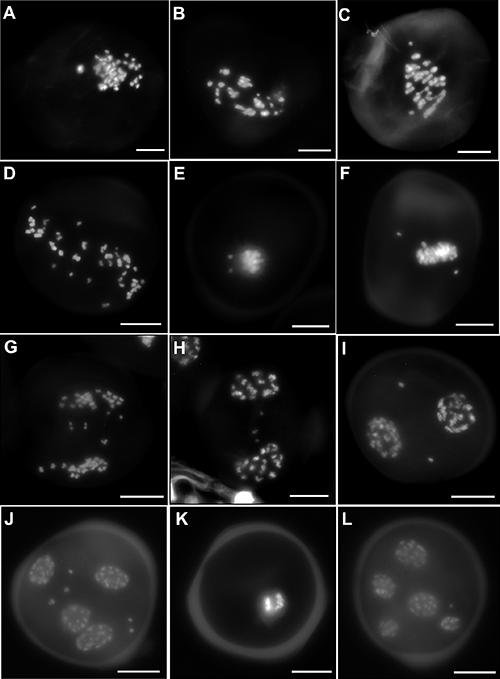
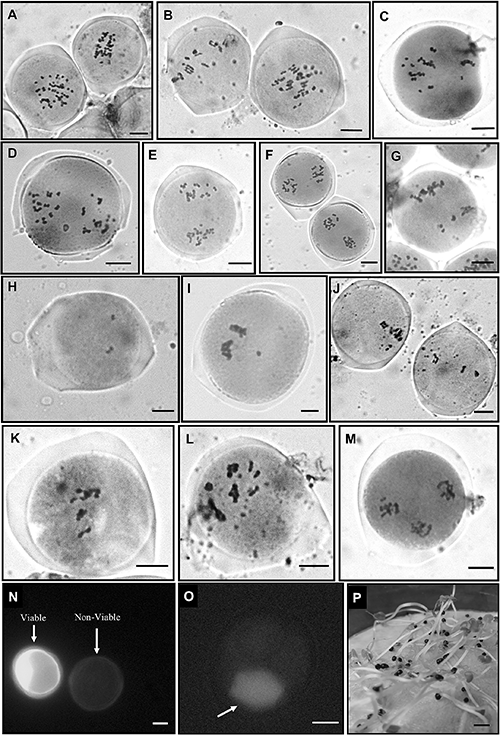
| Number of chromosomes | Percentage of PMCs (%) |
|---|---|
| 5–10 | 4.69 |
| 11–20 | 8.27 |
| 21–30 | 9.16 |
| 31–40 | 12.72 |
| 41–48 | 65.16 |
*More than 500 cells were analyzed to obtain this result.
The RA content in the leaves of C. scutellarioides has been quantified by HPLC. The average retention time of the RA in standard (Fig. 4A) and methanolic extract of the sample (Fig. 4B) is 16.956 min at 230 nm. The leaves contain 23.6±2.37 mg g−1 dry weight (DW) of RA.
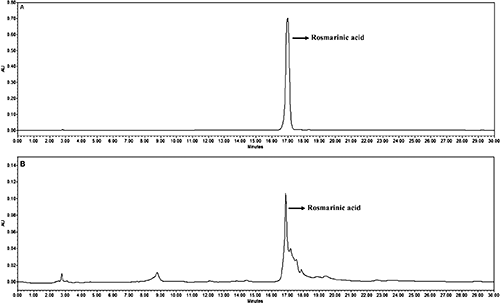
The members of the genus Coleus show considerable variations in chromosome numbers and it is suggested as polybasic having x=12, 14, 15, 16, 17, and 18 (Ramachandran 1967, Bir and Saggoo 1985). In the present study of C. scutellarioides, 48 chromosomes were found in the somatic cells and so based on x=12 the plant is tetraploid. The chromosome number and the tetraploid nature of the plant in the present study agree with the majority of cytological records (Table 1) (Bir and Saggoo 1985). Chromosomes are small and classified into metacentric and submetacentric only. The karyotype is therefore symmetrical and belongs to the 2B class of Stebbins’ asymmetry index (Stebbins 1971). All three pairs of submetacentric chromosomes have intercalary secondary constrictions. To the best of our knowledge, the detailed karyotype study in C. scutellarioides is not well documented. Consequently, the number and nature of secondary constrictions are remained unresolved. The range of chromosome length and types of C. scutellarioides has considerable similarities with the members of relative species of the genus such as Plectranthus neochilus, P. grandisand, P. barbatus, etc. though they greatly differ in the chromosome numbers (Nani et al. 2015, Reis et al. 2015).
Secondary associations between bivalents and the formation of multivalents during meiosis can be observed in segmental allopolyploids (Stebbins 1947, Gupta and Roy 1973, Mendes-Bonato et al. 2002). At present, a detailed study of meiotic division exhibits the presence of different valencies and secondary associations between the chromosomes indicating the segmental allopolyploid origin of this plant. The appearance of the multivalent chromosomes during meiosis has been reported earlier in this species suggesting the occurrence of polyploidy (Rife 1962, Hakeem and Rife 1966, Bir and Saggoo 1985). Multivalent formation and secondary association can lead to abnormal meiotic behavior that ultimately causes different kinds of meiotic irregularities like laggard chromosomes, irregular separation, multipolarity, etc. (Ortiz et al. 2011). An enormous number of PMCs of C. scutellarioides in the present study showing one to multiple laggards in different stages. This phenomenon is a result of the multivalent formation and secondary associations between bivalents. The normal PMCs found during the meiosis contain n=24 chromosomes in agreement with Bir and Saggoo (1985) and Mehra and Gill (1972). However, a striking feature found in the meiotic behavior is chromosomal mosaicism or variation of chromosome number among PMCs (Fig. 2H–L). The variation of chromosome number ranges from lower (<10 chromosomes) to higher order (>40 chromosomes). According to Reddy (1952), aneuploid chromosome number (n=24+1) has been recorded in the meiosis study, while we have found a large number of PMCs having numerical variations in chromosomes (Table 3). The aneuploid PMCs if survive and participate in sexual reproduction can be counted as a significant force that generates progenies with variant chromosome numbers. The numerical variation of chromosomes in the PMCs could be a correct explanation for this incident and may also be responsible for the existence of several garden forms or varieties in this species. Pollen viability in C. scutellarioides has been detected through the FCR of the FDA and found only 35.43% of pollens are viable. In the viable pollen, non-specific esterase hydrolyzes the FDA which led to the accumulation of the fluorescein in the cytoplasm that generates bright green fluorescence (Impe et al. 2020). Whereas the plasma membrane of the non-viable pollens is impaired that cannot retain the fluorescein so produces a ghost fluorescence. In allopolyploid plants, a massive number of nonviable pollens are expected due to the mispairing behaviors of chromosomes and the formation of multivalents and secondary associations (Ferreira de Carvalho et al. 2021). The high accuracy of this protocol also allows us to detect the viability of the cells in tetrads just after completion of the meiosis (Fig. 3O). In some cases, we have found that one or two cells are viable out of four cells in the tetrad which explain the existence of such low viability of pollen grains. Unlike the pollen viability, the seed germination rate in this species is satisfactory (87.18%). The high pollen sterility works as a selection to maintain the quality of viable pollens so that the fertilization process remains unaffected. As a consequence, the seed germination rate is good and produces healthy plantlets.
RA is a well-known phenolic compound having antioxidant, and antimicrobial properties along with several other medicinal activities. In several species of the genus Coleus such as C. amboinicus, C. aromaticus, C. blumei, C. forskohlii etc. the presence of RA is reported (Li et al. 2005, Kumaran and Karunakaran 2007, Bauer et al. 2015, Ślusarczyk et al. 2021). The leaves of C. scutellarioides are rich in RA estimated by HPLC and the amount of RA in the leaves is 23.6±2.37 mg g−1 DW. Though the RA has been measured in this species earlier with the treatment of LED light to accumulate 51.0±6.4 mg g−1 DW, their studies were not associated with the genetic characters (Dörr et al. 2019). A secondary metabolite is a dynamic array and changes with several environmental and genetic factors; therefore, the quantity of the natural product needs to be linked with the genetic characters of the plants (Kroymann 2011, Santra et al. 2021).
In the present study, we analyzed the detailed karyotype and meiotic behavior of C. scutellarioides, which received insignificant attention to date. The distinct meiotic behavior indicates the species is allopolyploid. In addition to that, anomalies like chromosomal mosaicism observed during meiosis became a major cause that increased the rate of abortive pollens. However, the seed germination rate is satisfactory. Finally, we also quantified the RA content having essential medicinal values in the leaves of C. scutellarioides through the HPLC technique. This is the first attempt where elaborate cytogenetical analysis in this plant is linked to the quantity of RA as a part of quality assessment. This detailed study will be valuable for the further development program of this ornamental medicinal plant.
IS and TH acknowledge the Government of West Bengal for providing the Swami Vivekananda Merit Cum Means Scholarship. All the authors acknowledge Swami Kamalasthananda, Principal, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata, India for the facilities provided for this study.