2023 Volume 88 Issue 1 Pages 41-44
2023 Volume 88 Issue 1 Pages 41-44
Cell walls in the giant-celled green alga Valonia are composed of multiple lamellae and designated as “crossed-fibril” type wherein each lamella contains cellulose microfibrils (CMFs) arranged in a uniform orientation. Outer, older lamellae are thinner than inner, younger lamellae, probably because the former are extended during cell growth more so than the latter without deposition of new cell wall materials. If this hypothesis is correct, the density of CMFs in the outer lamellae should be lower than that in the inner lamellae. In the present study, the density of CMFs in the outermost lamellae of the cell wall, in various sizes of Valonia utricularis growing cells, was calculated using images obtained with an atomic force microscope, and the densities were compared among the cells of different sizes. The CMF density ranged from 0.5 to 4.0 µm−1, which is lower than that previously recorded for the innermost lamellae (more than 10 µm−1). Moreover, the CMF density was negatively correlated with the cell size indicating that spacing between neighboring CMFs was increased by the cell surface extension due to cell growth. These findings may support the abovementioned hypothesis that the outer cell wall lamellae are extended by cell growth without the deposition of new CMFs.
A particular type of cell wall, called “crossed-fibril”, has been reported in some giant-celled green algae such as Boodlea (Mizuta and Okuda 1987), Chaetomorpha (Okuda and Mizuta 1987), Chamaedoris (Okuda et al. 1990) and Valonia (Mine et al. 2015). In these algae, cell walls are composed of multiple lamellae, in each of which the cellulose microfibrils (CMFs) are arranged in a uniform orientation, with the orientation differing by 80 to 120 degrees between neighboring lamellae (Mine et al. 2015). Ultrastructure observation of a section of the cell walls using a transmission electron microscope (TEM) has shown that the cell wall of Valonia utricularis is composed of many layers (Mine et al. 2018) wherein the lamellae near the outer side of the cell wall appeared thinner than those on the inner side. This is probably because the outer, older lamellae are subject to cell wall extension, caused by cell growth, for a longer time than inner, younger lamellae, without further deposition of cell wall materials from protoplasm.
On the other hand, the cell wall structure in terrestrial plants and Characean algae is characterized by “multinet” type (Probine and Preston 1961, Probine 1963, Giddings and Staehelin 1991, Mine et al. 2015). Within this structure, CMFs are first deposited in the innermost lamella of the cell wall, uniformly, in a radial orientation, and thereafter the orientation of the CMFs gradually becomes oblique due to longitudinal cell growth as the microfibrils are pushed into the outer cell wall lamellae by the newly deposited innermost lamellae. However, the changes in CMF arrangement in the crossed-fibril cell walls during expansive growth of the cell have not been investigated.
Supposing that outer cell wall lamellae extend in line with cell growth without the addition of CMFs in crossed-fibril cell walls, as inferred above, then it would be probable that the density of CMFs becomes lower with time and, therefore, CMF density in older lamellae would be lower than that in younger lamellae. In the present study, the spacing between CMFs in the outermost lamellae of the crossed-fibril cell wall in the cells of V. utricularis, grown to various sizes after cell division, was measured by observation using an atomic force microscope (AFM). We also compared the density of the CMFs in the outermost lamellae with that in the innermost lamellae and among cells of different sizes.
A unialgal culture strain of Valonia utricularis (Roth) C. Agardh, established from plants collected in Jhongliao, Green Island, Taiwan, on May 20, 2012, was used. The algae were cultured in a half-strength PES medium (Provasoli 1966) and kept under 12 h : 12 h light : dark cycles with 4 W m−2 illumination, provided by cool fluorescent lamps, at 20±2°C.
AFM observationsOne of the cells in the youngest generation of a thallus located on the distal end of the thallus (Fig. 1A) was isolated from the parent cell by excising it with a part of the parent cell using a razor blade. After taking an image of the isolated cell under a dissecting microscope to measure its size, the tip region of the cell, which is known to undergo isotropic growth immediately after cell division (unpublished data), was excised using a razor blade. The excised cell fragment was transferred to filtrated seawater (FSW) and the cell wall was isolated by removing the protoplasm using a hair of a paintbrush under a dissecting microscope. The isolated cell wall was placed on a coverslip with a drop of FSW so that the outside of the cell wall faced upward, before being air-dried after excess water was removed using tissue paper.
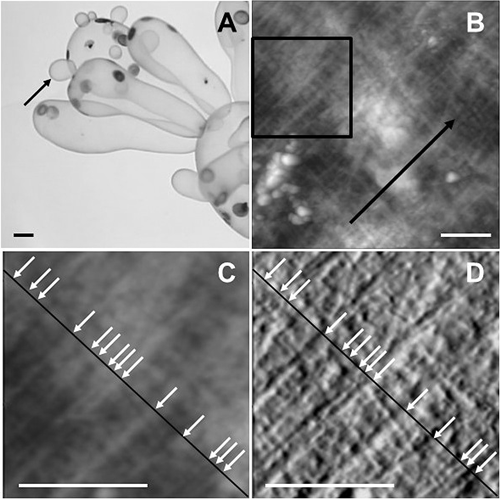
The surface of the isolated cell wall was then observed using an AFM (MFP-3D-SA, Oxford Instruments Asylum Research, Tokyo) equipped with a probe (OMCL-AC160TS, Olympus, Tokyo) in AC mode. Topographic and amplitude images (256×256 px; Fig. 1B) were obtained by scanning a 5×5 µm specimen area at a scanning speed of 0.5 s per line. Since it is known that the outer cell wall surface is mostly covered with amorphous materials preventing the visualization of the CMFs (Eslick et al. 2014), only the outer surface of the cell without the amorphous materials was observed in the present study.
Calculation of CMF densityIn a topographic image obtained using an AFM, specimen surface height is expressed by grayscale whereas the differential of the changes in height during line scanning is expressed in the amplitude image. A 2×2 µm area where the CMFs in the outermost lamellae were observed most clearly was selected and a line segment perpendicular to the CMFs was drawn (Fig. 1C, D). The presence of CMFs was confirmed when a filamentous structure perpendicular to the line segment was observed in both the topographic and amplitude images, and the density of CMFs was obtained by division of the number of CMFs intersecting the line segment by the length of the line segment.
In the present study, a cell of the youngest generation of the thallus (Fig. 1A) in each of 11 thalli of V. utricularis that had been separately cultivated was used to calculate CMF density. The size of the 11 cells was 1.1–2.9 mm in length and 0.73–1.62 mm in width. AFM image was obtained in each of three different areas in a cell wall fragment isolated from each cell, the density of CMFs in the outermost lamellae was calculated for each of the three AFM images per cell, with the average of the three calculations being recognized as the CMF density of the cell. The CMF density ranged from 1.5–3.2 µm−1, whereas the density of the CMFs in the inner surface of the cell wall has previously been shown to be more than 10 µm−1 (Mine and Sekida 2018). Therefore, the CMFs in the outermost lamellae are sparser than in the innermost lamellae.
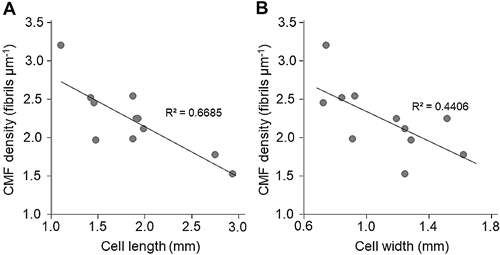
The graphs in Fig. 2 represent the relationships between the density of CMFs in the outermost lamellae of the cell wall and cell size (length and width of the cells). If the density of CMFs is decreased by cell growth, it would be expected that CMF density is lower in larger, more mature cells than in smaller cells. As expected, CMF density was negatively correlated with the cell size (Fig. 2) and the correlation coefficient (R2) between CMF density and cell length was 0.669, and that between CMF density and cell width was 0.441. Thus, the spacing between CMF in the outermost lamellae of the growing cell of V. utricularis was greater in larger cells than in smaller cells.
In the present study, it was found that the density of CMFs in the outermost lamellae of the cell wall in the growing cells of V. utricularis was mostly less than 3 µm−1. The CMF density in the inner surface of the cell wall has previously been shown to be more than 10 µm−1 (Mine and Sekida 2018). Therefore, the CMFs in the outermost lamellae are sparser than in the innermost lamellae. This finding implies that the lamellae of crossed-fibril cell walls, as with those of multinet cell walls, extend in correspondence with cell surface extension during cell growth without the addition of cell wall materials from protoplasm, supporting the abovementioned hypothesis based on the TEM observations of Mine et al. (2018).
This is further supported by the finding in the present study that CMF density was lower in larger cells than in smaller cells because the cell surface is extended more in the former than the latter causing the separation of neighboring CMFs in the cell wall. Furthermore, the fact that CMF density was negatively correlated with cell size would imply the possibility that the outermost cell wall lamellae persist along the surface of cell wall without falling off until the cells grow into the size of the cells used in the present study.
As seen in Fig. 1B–D, the sparse distribution of CMFs in the outermost cell wall lamellae was uneven; some CMFs were closely associated with small spaces between them, whereas others were arranged with neighboring CMFs but with wider intervening spaces. In the cell walls of V. utricularis, fibrous matrix components were previously reported to coil around the individual CMFs, and some of them seemed to bundle multiple CMFs (Mine and Sekida 2018). Although the mechanical interaction between CMFs and the matrix components has not been clarified, there is a possibility that the fibrous matrix components play a role in maintaining close associations between neighboring CMFs in the outermost lamellae of the cell wall.
We thank Ms. Miho Tokimitsu, Department of Biology, Faculty of Science, Kochi University, for technical assistance during the present study.