2023 Volume 88 Issue 2 Pages 143-146
2023 Volume 88 Issue 2 Pages 143-146
Apteronotus include a large number of recognized species, but few have been cytogenetically studied. This study establishes the first cytogenetic description of A. ellisi collected from the upper Paraguay River basin, which presented 2n=52 chromosomes, karyotype composed of 20 metacentric, 20 submetacentric, eight subtelocentric and four acrocentric chromosomes, and fundamental number as 100 for both sexes. Heteromorphic sex chromosomes were absent. A pair of nucleolar organizing regions (NORs) was detected in the submetacentric chromosome pair 13 by silver-staining. Heterochromatic regions were observed in the long arms of the NOR-bearing chromosome pair. Besides the present data are valuable to help in understanding karyotypic evolution in Apteronotidae. Data from NORs confirmed the tendency of this family in presenting simple NORs sites, similar to the other Gymnotiformes clades. Yet, the presence of little heterochromatin can be used as cytogenetic markers for A. Ellis, and centric fusions/fissions appear to be an important mechanism in the karyotype evolution and differentiation among Apteronotus species.
Fish constitute an extremely favorable group for cytogenetic and evolutionary studies since they occupy a basal position in the vertebrate phylogeny (Nelson 1994). In the Upper Paraguay Basin, there are approximately 400 species of fish, of which 263 species are cataloged for the Pantanal (Britski et al. 1999, Reis et al. 2003). These constitute important ecological resources for this region as a biotic compartment of the system, as they constitute seasonal food around which vertebrate species aggregate.
Apteronotidae is an assemblage of high species diversity, which are well known for their electrogenic abilities, presenting 94 valid species (Albert and Crampton 2005). Apteronotids are found in rivers from Panama to Northern Argentina, including rivers that flow into the Pacific Ocean, and in the Orinoco, Maracaibo, Magdalena, Guyana shield, Amazon, Paraná-Paraguay and San Francisco River basins (Albert and Crampton 2005). In Apteronotidae, the species of the genus Apteronotus are the most widely distributed, inhabiting floodplains, streams, and rivers (Albert 2001). A. ellisi is distributed in the Paraná and Paraguay basins, presents a body elongated and compressed, ground color dark-brown dorsally, posterior portion of caudal peduncle and caudal fin base with light-beige blotch, caudal fin dark-brown (Ota et al. 2018).
Apteronotus include a large number of recognized species, but few have been cytogenetically studied. Despite this fact, they have shown highly diverse karyotypes in terms of differences in both 2n and karyotype structures, with the 2n ranges from 2n=24 in A. albifrons (Howell 1972, Almeida-Toledo et al. 1981, Mendes et al. 2012, Fernandes et al. 2017) to 2n=52 in Apteronotus sp. (Almeida-Toledo et al. 2007), including the presence of B chromosomes in genomes of A. albifrons (Mendes et al. 2012).
To collect more information about chromosomal diversity within the family Apteronotidae, this study establishes the first cytogenetic description of A. ellisi by classic cytogenetics techniques.
Specimens of A. ellisi were collected from the Onça Stream, upper Paraguay River basin (Coxim–MS; 18°30′24.34″S/54°40′36.39″W). The individuals were identified and deposited in the Universidade Estadual do Mato Grosso do Sul. The experiments followed ethical conduct, and before sacrificing, fish were anesthetized by an overdose of clove oil (Griffiths 2000). Metaphase chromosomes were obtained from anterior kidney cells using the air-drying technique (Bertollo et al. 1978). The C-positive heterochromatin (C-bands) was visualized following the procedure by Sumner (1972) and stained with propidium iodide (Lui et al. 2012). NORs were detected by silver nitrate staining according to Howell and Black (1980).
At least 30 metaphases were analyzed for each specimen and those with good chromosome morphology were used for the karyotype analyses. Chromosomes were classified as metacentric (m), submetacentric (sm), subtelocentric (st), and acrocentric (a) according to the ratio between long arm (q) and short arm (p) (Levan et al. 1964). For the determination of the fundamental number (FN), that is, the number of chromosome arms, the m, sm, and st chromosomes were calculated as bearing two arms and the a chromosomes one arm.
The chromosomes were analyzed in an epifluorescence microscope Olympus BX51 and the images were captured using the software DP controller (Media Cybernetics), and image composition was carried out with Adobe Photoshop CS6.
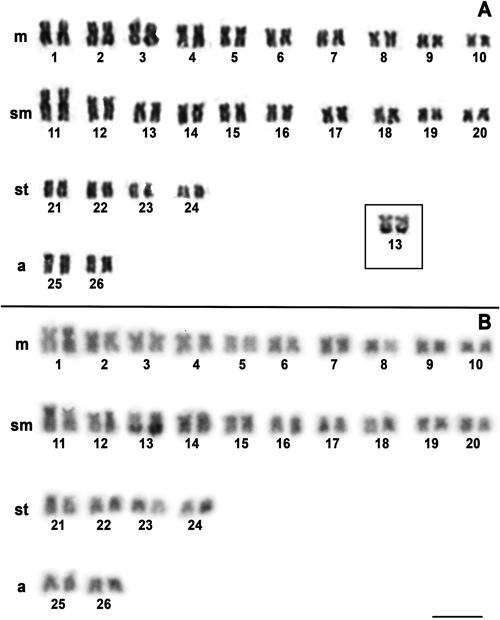
The individuals of A. ellisi had 2n=52 chromosomes and karyotype composed of 20m, 20sm, eight st, and four a, and FN=100 for both sexes (Fig. 1A). Heteromorphic sex chromosomes were not found. Ag-NOR sites were located on a chromosome pair in the karyotypes of all individuals. These sites were located at the terminal position on the q arm of pair 13. C-band was detected on a few chromosomes, in the pericentromeric region in pair 14, and evident blocks located in the terminal position on the q arm of pair 13, coincident with the marking Ag-NOR (Fig. 1B).
The lack of karyotype data for several fish groups impairs comparative analyzes of their evolutionary trends and chromosomal relationships. This is the case for the Apteronotus for which chromosomal characteristics are known only for three species: A. albifrons with 2n=24 chromosomes (Howell 1972, Almeida-Toledo et al. 1981, Mendes et al. 2012, Fernandes et al. 2017), A. caudimaculosus with 2n=26 chromosomes (Fernandes et al. 2017) and Apteronotus sp. with 2n=52 chromosomes (Almeida-Toledo et al. 2007). In this sense, this study is the first one providing classical cytogenetic data for one of its representative species, A. ellisi.
Both male and female specimens of A. ellisi have the same karyotype structure, with 2n=52 chromosomes (20m+20sm+8st+4a), with no evidence of differentiated sex chromosomes. The diploid number of 52 chromosomes found in A. ellisi differs from the findings for A. albifrons (2n=24) and A. caudimaculosus (2n=26), however, and the same number finds for Apteronotus sp (2n=52). Considering our results, A. ellisi only is the fourth karyotyped species.
Although having the same 2n=52, the individuals of A. ellisi under study differed in their karyotypes and FN values of Apteronotus sp (Almeida-Toledo et al. 2007). This difference was mainly due to the number of subtelocentric/acrocentric chromosomes—12 (FN=100) from the A. ellisi and six (FN=98) from the Apteronotus sp. Thus, the similar karyotypic macrostructure between A. ellisi and Apteronotus sp. probably indicates that these species are undergoing a recent speciation process. In turn, compared with other Apteronotus possessing 2n=24 (A. albifrons) and 26 (A. caudimaculosus), the karyotype of A. ellisi suggested an increase of 2n due to chromosome fission, indicating that chromosome rearrangements, such as centric fusions/fissions that can alter the chromosome number may have occurred during the diversification of this group.
NORs were located in the terminal position on the q arm number 13, as revealed by the Ag-NOR technique, thus characterizing a simple NORs system in A. ellisi. A similar pattern was observed in karyotypes of A. albifrons populations (Almeida-Toledo et al. 1981, Mendes et al. 2012, Fernandes et al. 2017) and Parapteronotus hasemani, Sternarchogiton preto, Sternarchorhamphus muelleri (Silva et al. 2014). However, in the karyotype of A. caudimaculosus, in additionally to Ag-NORs sites, 18S rDNA clusters were detected also in other two chromosome pairs, featuring multiple NORs systems (Fernandes et al. 2017). Apteronotus sp. did not show NOR data (Almeida-Toledo et al. 2007). According to already published data, simple Ag-NORs phenotypes were observed for all Apteronotidae species (Almeida-Toledo et al. 1981, Mendes et al. 2012, Silva et al. 2014, Fernandes et al. 2017), except, multiple cistrons of 18S rDNA observed for only A. caudimaculosus (Fernandes et al. 2017). Thus, a single chromosome pair with NORs sites observed in A. ellisi is thought to be a plesiomorphic characteristic of the Apteronotidae.
C-band was detected on a few chromosomes in A. ellisi, where conspicuous large blocks were prominent/remarkable on the q arm of pair 13, coincident with the marking Ag-NOR. On the other hand, karyotypes of the individuals of A. albifrons from the Paraná River basin (Fernandes et al. 2017) had large C-bands in the NOR-bearing chromosome, adjacent to secondary constriction, but the NOR sites themselves were not C-band positive. In turn, karyotypes of individuals of A. albifrons from the Amazon basin (Almeida-Toledo et al. 1981) and those from the Paraná basin (Fernandes et al. 2017), as well as A. caudimaculosus from the Paraguay basin (Fernandes et al. 2017) had a contrasting pattern to A. ellisi, with a large number of positive C-bands distributed over the pericentromeric region of several chromosomes. Thus, the distribution of C-band can be useful as a cytotaxonomic marker among Apteronotus, separating at least two groups, those with C-banded in the pericentromeric region of several chromosomes (A. albifrons and A. caudimaculosus) and those with few C-band (A. ellisi) spread throughout the genome.
The results presented in this study allowed the first cytogenetic characterization of A. ellisi, contributing to the karyotype knowledge of the family Apteronotidae. Data from NORs confirmed the tendency of this family in presenting simple NORs sites, similar to the other Gymnotiformes clades. We also reported the presence of a few C-bands, with C-band in the NOR-bearing chromosome, that can be used as cytogenetic markers for A. ellisi, and that centric fusions/fissions appear to be an important mechanism in the karyotype evolution and differentiation among Apteronotus species.
The authors thank Dr. Fernando Carvalho da Universidade Federal de Mato Grosso do Sul for the taxonomic identification of the specimens.