2023 Volume 88 Issue 3 Pages 265-272
2023 Volume 88 Issue 3 Pages 265-272
The popular ornamental flowering plant Dianthus hybrida cv. Telstar Scarlet has been found to exhibit two populations, each with distinct flower morphology: female-like and hermaphroditic. In this work, flower development of D. hybrida was characterized through scanning electron microscopy, light- or stereo- microscopy, from flower meristem formation to the fully matured, open flower. The difference between hermaphrodite and female-like plants was initially marked by the phenomenon of anther shrinking in the latter, which was closely associated with pollen shrinking phenomenon, and soon followed by differential elongation rates of pistils and stamens. Furthermore, the female-like anther, albeit exhibiting delay in pollen development initially, could produce microspores, resembling its hermaphrodite counterparts at some point, before shrinking. However, female-like’s pollen and cell wall size never became as large as the hermaphrodite’s.
Dianthus hybrida, an interspecific hybrid of D. chinensis and D. barbatus, is a popular ornamental flowering plant. There are two distinct populations found in one cultivar ‘Telstar Scarlet’: female-like and hermaphroditic. Female-like and hermaphrodite plants have comparable overall flower sizes to one another. A fully developed flower has a calyx of five fused sepals and a corolla of five free petals, whose positions alternate with those of sepals, and pistil readily visible, as well as possessing 10 stamens. Nevertheless, in female-like flowers, stamens filaments are relatively shorter, with their anthers wrinkled, possibly rendering its pollen infertile.
Whether male sterility is occurring in D. hybrida cv. Telstar Scarlet female-like population has not been reported and may arise from interspecific hybridization (Liao et al. 2020), parental lines inheritance, or simply spontaneous mutations (Kinoshita et al. 1982). Regardless, the phenomena where female and hermaphrodite populations are present in one species is called gynodioecy, and it occurs relatively frequently among Caryophyllaceae, to which Dianthus [e.g., D. shinanensis, D. superbus (Kuhara and Sugawara 2002), D. pavonius (Bruns et al. 2019)] and the dioecious Silene latifolia belong.
Ornamental plants may undergo future genetic modification by transgene introduction. In D. hybrida, transcriptome analysis to identify genes working at a certain environmental condition was also performed (Nishijima et al. 2022). When producing a modified plant of a certain gene, it is in our interest to develop transgenic plants that is infertile to reduce the chance of inadvertent spread of transgenes which, in turn, has the potential to significantly alter the ecological balance within a short time frame (Pilson and Prendeville 2004). Therefore, one objective of this research is to investigate the fertility of pollen in both female-like and hermaphrodite plants.
Major flower developmental events of Dianthus distinguishing hermaphrodite and female plants have been described, starting to noticeably differ through unaided eyes a few days before anthesis (Bruns et al. 2019, Hawkins 2019, Kuhara and Sugawara 2002). However, unlike S. latifolia, whose female flower is known to lack stamen primordia since the very beginning of its development (Kazama et al. 2022), the earliest step at which Dianthus hermaphrodite and female flower development start to diverge, as well as a detailed flower and pollen morphological development is unknown. This difference can be a cause of the loss of male function in this species.
In this paper, we observed the structure of D. hybrida flower buds by scanning electron microscopy and stereo microscopy from their first appearance as a flower meristem until they open as fully developed flowers. For presentation, we have divided the steps of floral development into stages considering those defined for the model plant Arabidopsis thaliana (Smyth et al. 1990), as well as the dioecious Silenoidea S. latifolia flower (Grant et al. 1994, Farbos et al. 1997), and defined new developmental stages when needed (Table 1). By 4′,6-diamidino-2-phenylindole (DAPI), 2,3,5-triphenyltetrazolium chloride (TTC) and toluidine blue staining followed by light- or fluorescence- microscopy, some aspects of pollen and tapetum development were also revealed.
| Stage no. | Morphological description | |
|---|---|---|
| Hermaphrodite | Female-like | |
| 4 | Five sepal primordia emerged. Dome-shaped flower primordia. | |
| 5 | Emergence of stamen primordia. | |
| 6 | Pistil primordium appeared. | |
| 7 | Five early emerged stamen “long stamen” primordia differentiated into filaments and anthers. | |
| 8 | Anthers theca became obvious. | |
| 9 | Petals grew, with length still below the short stamens. | |
| 10 | Petal length was about equal to the short stamen. | |
| 11-1 | Petal length was in between the short- and long-stamens. | |
| 11-2 | Petal length was about equal to the long stamen. | |
| 11-3 | Petals were about to enclose the reproductive parts. | |
| 11-4 | Petals completely enclose the reproductive parts. | |
| — | Anthers shrunk. | |
| 11-5 | The flower bud expanded further. | |
| Anthers showed red pigmentation. | — | |
| 11-6 | Petals started to turn red. | |
| 11-7 | Petals completely turned red. | |
| 11-8 | Flower opened a bit. | |
| Pistil cannot be seen. | Pistil was visible. | |
| 12-1 | Flower opened further (∼45° angle). | |
| Only 5 stamens were visible. | — | |
| 12-2 | Flower completely opened (∼90° angle). | |
| All 10 stamens became visible. | — | |
| Pistil is now visible. | ||
| 12-3 | Pistil distal end curled. | |
D. hybrida cultivar ‘Telstar Scarlet’ seedlings were maintained and stem cutting-propagated on soil or agar media in an incubator at 23°C, 16-h light/8-h dark cycles.
Scanning electron microscopy and stereo microscopyFreshly picked flower buds were first uncovered from their leaf sheaths using a tweezer under a stereo microscope. The buds were then observed using a scanning electron microscope (FlexSEM 1000II, Hitachi Ltd., Tokyo, Japan), operated at 5 kV, with a cool stage at −20°C, or a stereo microscope Leica M205 FA (Leica Microsystems, Tokyo, Japan).
Pollen observationUpon removing freshly picked flower buds/flowers’ leaf sheath (and sometimes, its sepal, petal, and/or pistil), the stamens (anther) were isolated and placed onto slide glass on top of which a few drops of DAPI solution (Slow Fade™ Diamond Antifade Mountant with DAPI; Invitrogen by Thermo Fisher Scientific, Oregon, USA) was added. Next, the anther was disturbed using a fine point tweezer to release its tissue content (i.e., pollen). After placing a cover glass on top of the sample, incubation was allowed in the dark at 4°C overnight. A fluorescence microscope Zeiss AXIO Imager A2 was used to observe the prepared samples. The microscope and camera settings such as lighting intensity and exposure time were set to be equal across all studied samples.
The developing flower buds were observed using a scanning electron microscope (Fig. 1). So far, we have not been able to find the corresponding stages 1 to 3 flower bud, which is characterized by elongated (oval) floral meristem, based on a previous study using S. latifolia (Grant et al. 1994). The emergence of all five sepal primordia underneath the dome-shaped flower meristem marked the starting point of stage 4, followed by stage 5, the emergence of stamen primordia. In stage 6, pistil primordium arose. Stage 7 is characterized by the differentiation of five early-developed stamen primordia into filaments and anthers. From this stage and beyond, sepals had been removed to allow observation of inner structures. In stage 8, anthers theca became obvious. Unlike S. latifolia whose differences between male and female flowers are noticeable from stage 6 (Grant et al. 1994, Farbos et al. 1997), the morphological differences between the hermaphrodite and female-like flower in D. hybrida were not obvious until later stages of development.
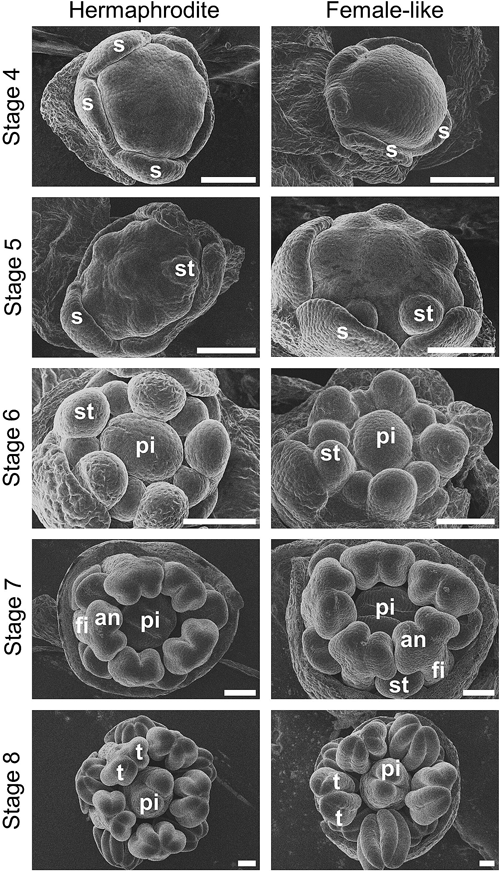
Stage 4: five sepals (s) underlay flower meristem. Stage 5: Stamen (st) primordium arose. Stage 6: pistil (pi) primordium emerged. Stage 7: long stamen primordia separated into filament (fi) and anther (an); pistil formed cleft; sepals were removed to reveal inner structures. Stage 8: anthers’ theca (t) was defined; pistil elongated by emerging styles. Scale bars=100 µm.
From stage 9 to stage 12, flower observation was conducted using a stereo microscope (Figs. 2, 3). In stage 9, the whole flower buds appeared translucent; petal primordia grew further to overlay the lower set of stamens—stamen with short filament—hereafter termed “short stamen.” Stage 10, meanwhile, was defined by the petals reaching the short stamens in length. Stage 11, which consists of the flower bud’s further growth until it is just about to open, is divided into eight steps. In stage 11-1, the petal length was in between the short stamen and long stamen; while, in stage 11-2, the petal length, at last, became equal to stamens with long filament, hereafter termed “long stamen.” In stage 11-3, petals were about to enclose the reproductive parts, whereas in stage 11-4, petals completely covered it. Stage 11-4 marked the starting point of a clear difference between the hermaphrodite and female-like flower: the latter has its anthers wrinkled and shrunk (Fig. 2, Fig. S1). This anther shrinking phenomenon might occur a bit earlier or later than stage 11-4, but it happened most frequently at this stage.
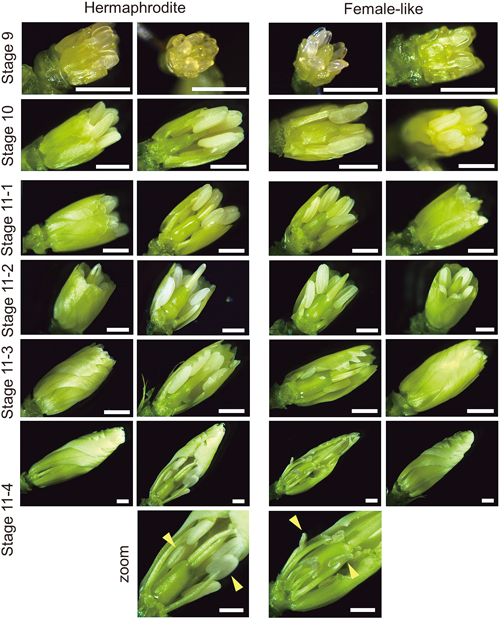
Stage 9: petal primordia stalked at the base. Stage 10: petal level with short stamens. Stage 11-1: petals about to level with long stamens. Stage 11-2: petal level with long stamens. Stage 11-3: petals about to enclose the bud. Stage 11-4: petals enclosed bud; zoom: enlarged view of the stamens (anther—yellow arrowhead) of stage 11-4 flowers; female flower anthers shrunk. Some petals and stamens might have been removed to reveal the pistil. Scale bars=1 mm.
In stage 11-5, the entire bud expanded further (Fig. 3). However, flowers from female-like plants appeared to have shorter stamen filament. On the other hand, stamen filament in hermaphrodite flowers was relatively longer and its anther began to display red pigmentation. In stage 11-6, the petals started to turn red, while complete red pigmentation of the petals characterized stage 11-7. Concomitantly, we also noticed that the pistil was longer in female-like flowers. In stages 11-8 to 12-1, flower bud (petals) was in the process of opening. During these stages, the pistil could not be seen easily without dissecting the flower in a hermaphrodite plant, while in a female-like flower, a pistil could be immediately seen as soon as the flower is open. Also notice that, at first, the number of readily visible stamens in hermaphrodite flowers was just five. Only after a while since the hermaphrodite flower was fully open, its pistil (stigma and style), as well as the five remaining stamens, extended further to be visible (stage 12-2). Meantime, stamens never become readily visible in female-like flowers. Finally, a completely mature, open flower of D. hybrida was defined by the presence of extensive curling at the distal end of the pistil in both hermaphrodite and female-like flowers (stage 12-3). Therefore, D. hybrida flower development appears to be comparable to D. pavonius (Hawkins 2019), D. shinanensis, and D. superbus (Kuhara and Sugawara 2002).
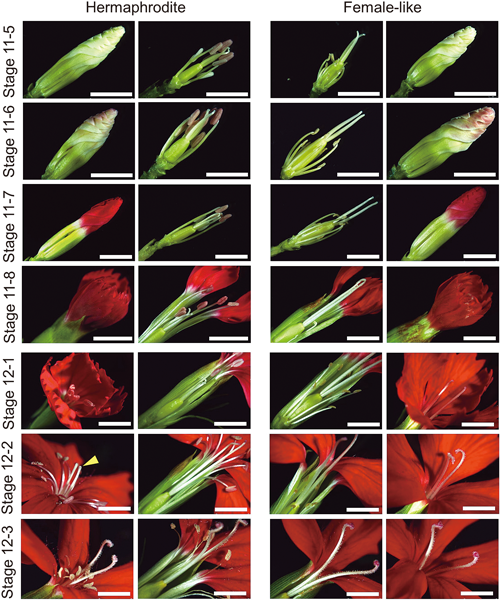
Stage 11-5: floral organs extended further, except for female-like flower stamen filament whose extension was minimal. Stage 11-6: petals started to turn red. Stage 11-7: petals completely pigmented. Stage 11-8: flower about to open. Stage 12-1: early open flower; pistil of hermaphrodite flower had not reached the maximum length. Stage 12-2: hermaphrodite flower pistil extended further that it now can be seen immediately (yellow arrowhead); Stage 12-3: pistil of both flower types reached the maximum length and its style curled. Scale bars=5 mm.
As anther shrinking was one striking difference in flower development between hermaphroditic and female-like plants, we decided to investigate the pollen that it contains. Pollen grains from various stages of flowers were characterized by fluorescence microscopy upon its release from anther by mechanical disruption using a tweezer and subsequent incubation in DAPI solution (Fig. 4, Fig. S2).

For each flower stage, pollen grains were either derived from (S) short stamen or (L) long stamen. Fresh pollen grains were obtained from mechanically disturbed anthers of flower stages 10 to 11 and then stained with DAPI overnight before observation. Scale bars=10 µm. See also Table 1.
Flowers of stage 10 has two distinct pollen populations. In female-like flower, those derived from five short stamens were at the tetrad stage, characterized by multiple discrete nuclei signals per cell; while those derived from five long stamens were at the ‘just-released’ microspore stage, characterized by single nuclei per cell and the relative lack of cell wall. In the case of hermaphrodite flower, pollen grains taken from short stamen were already beyond the ‘just-released’ microspore stage: the microspore cell wall seen there was thicker; while those from long stamen was at an even later microspore stage: the cell size was noticeable larger with its cell wall was even thicker.
Flowers of stage 11-1 also had two distinct pollen morphologies. In female-like flowers, pollen obtained from both long stamen and short stamen were at the microspore stage, however, the latter showed thinner cell walls than the former. In the case of hermaphrodite flowers, those from short stamens were at the microspore stage that resembles microspores from long stamens of stage 10, while microspores derived from long stamens became even larger than microspores of long stamens of stage 10, not to mention that its cell wall thickened more than ever. Microspores of the long stamen of stage 11-1 female-like flower were smaller than those of hermaphrodite flowers at the same stage and looked like microspores of the short stamen of stage 10 hermaphrodite flower.
From flower stage 11-2 onwards, pollen grains no longer displayed obvious distinction between those obtained from long-and short-stamen. Both hermaphrodite and female-like stamens contained an even larger microspore with an even thicker cell wall than those in the previous stage. However, microspores derived from female-like flowers were of smaller sizes than their hermaphrodite counterparts. Meanwhile, microspores originated from hermaphrodite flowers, starting from this stage, had no detectable nuclei DAPI signal.
In hermaphrodite flowers, pollen grains from flower stage 11-3 and stage 11-4 (at which anther started to shrink in female-like flower; see Fig. 3) did not show a noticeable morphological change than those observed in stage 11-2. In female-like flowers, pollen grains from stage 11-3 appeared to reach the maximum cell walls thickness and some exhibited less intense nuclei signals than those in stage 11-2; and in stage 11-4, the pollen boundary was no longer spherical: pollen grains get shrunk. Female-like pollen grains at this stage also displayed no detectable nuclei DAPI signal.
Overall, around stages 11-3 and 11-4, many cells of both hermaphrodite and female-like displayed no detectable nuclei signal: we were not sure whether the microspore had successfully undergone mitosis as the nuclei signals became less distinct over the developmental timeline. Nuclei-less pollen observed in late-stages flowers of both hermaphrodite and female-like plants might have been due to genetic incompatibility arising from interspecific hybridisation (Liao et al. 2020), preventing complete maturation of the microspore. To be noted that this lack of nuclei signal was not due to non-penetration of DAPI through the cell membranes since mature S. latifolia pollen nuclei DAPI signal could be seen by applying the same method (Fig. S3) and various other methods involving chemical fixation and/or enzyme treatment still could not produce nuclei DAPI signal (data not shown). Moreover, the pollen, regardless of whether they are from the female-like or hermaphrodite flower, was probably inviable as judged by TTC staining (Fig. S4) and the lack of seed-bearing carpels (data not shown).
We have considered several putative causes of the anther-pollen shrinking phenomenon. We observed that microsporocytes could transform into tetrads in female-like flowers (Fig. 4). Therefore, putative defect in meiosis during microsporogenesis might not be a factor that causes the degeneration and shrinking of microspores in female-like flowers later, just as it was suggested to be in the case of Asparagus officinalis (Ide et al. 2019). Another possible factor was ethylene, which is revealed to be produced in pistil and causes anther arrest in the flower of Cucumis species (Boualem et al. 2008, Manzano et al. 2016). Defect in proline biosynthesis (p5cs) has also been implicated in pollen shrinking and its nucleus DAPI signal degeneration in A. thaliana (Mattioli et al. 2012).
From the current observations, we concluded the loss of maturation of microspores had been associated with the shrinking anther phenomenon in female-like flowers, both of which occurred around flower stage 11-4. Although the identification of the gene responsible for this phenotype remains unknown, such a genetic mutation, when found, would be appropriate as an example of the cause of gynodioecy. Furthermore, the pollen-defective D. hybrida plant could be applied for genetic engineering and breeding of this plant as it does not pose the risk of escaping through pollen dispersal.
This research was supported by JSPS KAKENHI Grant Numbers JP22H05071 and JP21KK0128 to Y.K as well as Grant for Strategic Issue Research Promotion 03-04R020207 from Fukui Prefectural University to H.S. and Y.K.
A.S., R.N., and Y.K. designed research; A.S and T.K. performed experiments; A.S., H.S., and Y.K. drafted the text of the manuscript. All the authors contributed to writing and editing the manuscript.
Supplementary information including Figs. S1, S2, S3, and S4 is available online.