2024 Volume 29 Pages 34
2024 Volume 29 Pages 34
Background: Exposure to fine particulate matter (PM2.5) has been associated with allergic diseases, including asthma. However, information about the effects of specific PM2.5 components is limited. This study aimed to investigate the relationship of exposure to chemical components of PM2.5 during pregnancy and early childhood with the development of asthma, allergies, and sensitization in school-age children.
Methods: This study included 2,408 children in the second grade of elementary school. Questionnaire surveys of respiratory/allergic symptoms and measurements of serum total IgE and specific IgE levels to house dust mite (HDM) and animal proteins were conducted. Exposures to ambient PM2.5 mass, sulfate (SO42−), nitrate (NO3−), ammonium (NH4+), elemental carbon (EC), and organic carbon (OC) of PM2.5 in participants’ residences from conception to age six were estimated using predictive models. Multiple logistic regression analysis was used to analyze the association of respiratory/allergic symptoms and allergen sensitization with estimated exposure concentrations, after adjustment for survey year, sex, season of birth, feeding method during infancy, presence of siblings, history of lower respiratory tract infection, use of childcare facilities, passive smoking, presence of pets, mother’s age, history of allergic diseases, smoking during pregnancy, and annual household income.
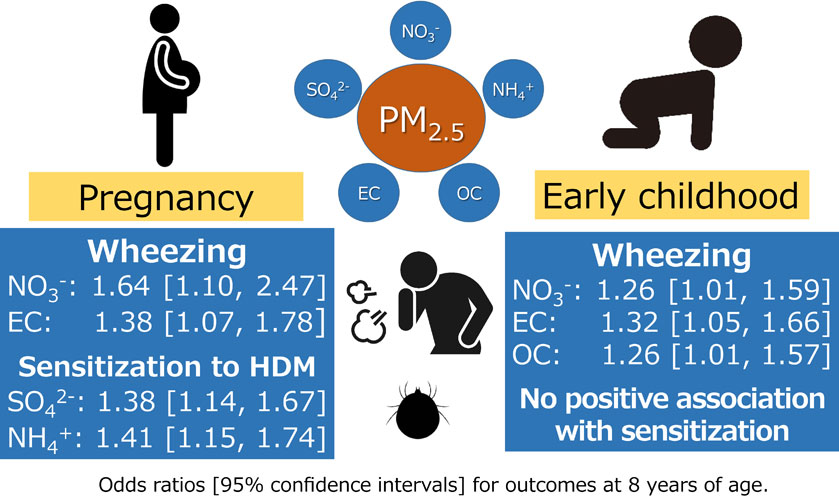
Results: No significant association was found between PM2.5 and its component concentrations and asthma. However, wheezing significantly increased with mean NO3− concentrations during pregnancy (odds ratio of 1.64 [95% confidence interval: 1.10, 2.47] for an interquartile range increase). Significant associations were also found between EC in the second trimester of pregnancy and PM2.5, NO3−, EC, and OC concentrations in early childhood. Higher PM2.5, SO4−, and NH4+ concentrations during the second trimester increased the risk of rhinitis. Sensitizations to HDM and animal proteins were significantly associated with exposure to components such as SO42− and NH4+ during pregnancy but not with postnatal exposure.
Conclusions: Exposures to NO3−, EC, and OC during pregnancy and early childhood were associated with wheezing. SO42− and NH4+ exposures during pregnancy were associated with sensitization to HDM and animal proteins. Asthma was not associated with exposure to PM2.5 and its main components at any period.
In recent years, the prevalence of allergic diseases has been on the rise among children. Both genetic [1] and environmental [2] factors are involved in this increase. Additional variables such as region, lifestyle, dietary habits, socioeconomic status, climate, and medical systems may be involved [3]. Notably, exposure to air pollutants is one of the most important environmental factors contributing to the development of allergies and asthma [4].
The influence of ambient fine particulate matter with a particle size less than 2.5 µm (PM2.5) has garnered increased attention [5–7]. PM2.5 is considered to permeate the maternal alveoli and placental barrier during the fetal period and directly affect the fetus [8]. During the fetal period, the lungs are immature and thus susceptible to environmental factors [9]. Many birth cohort studies conducted in Western countries have reported an association between exposure to air pollutants, including PM2.5, during the fetal period and early childhood and the onset of wheezing and asthma in childhood [5, 6, 10, 11]. A meta-analysis of these studies revealed that maternal exposure to PM2.5 during pregnancy was associated with wheezing and asthma in children up to three years of age [12]. Additionally, it has been reported that exposure to air pollutants, such as PM2.5, poses a risk of allergic rhinitis and allergen sensitization in children [13–15]. In contrast, a meta-analysis of five European birth cohort studies found no association between exposure to air pollutants, including PM2.5, in the prenatal and postnatal periods and childhood asthma, rhinoconjunctivitis, or allergen sensitization [16, 17]. These findings indicate the absence of a definitive conclusion regarding the association between exposure to air pollutants in the prenatal and postnatal periods and childhood allergic diseases such as asthma.
Ambient PM2.5 comprises carbon and various ion species [18]; however, the components that affect health have not been identified. A birth cohort study in the United States reported an association between ambient nitrate (NO3−) exposure during the fetal period and the onset of asthma in 6-year-old children [19]. Similar observations in Canada and China demonstrated that exposure to black carbon (BC), NO3−, and other components during the fetal period and early postnatal periods increased the incidence of childhood asthma [20, 21]. However, the features of PM2.5, including concentration, composition, and source, vary significantly depending on the region. While ambient PM2.5 concentration in Japan has shown improvement [22], it still exceeds the guideline level for PM2.5 (annual mean value of 5.0 µg/m3 or less) revised in 2021 by the World Health Organization (WHO). The effects of PM2.5 exposure during the prenatal and postnatal period on children have been reported even in the United States [19], Canada [20], and Australia [23], where levels of air pollution are lower than in Japan. However, little is known in Japan about the effects of air pollutant exposure during the fetal period, and there is little knowledge about its association with specific components.
Evaluations based on birth cohort studies are preferable for elucidating the influence of environmental factors on childhood allergic diseases such as asthma. The Japan Environmental and Children’s Study (JECS), a birth cohort study conducted by the Ministry of the Environment of Japan, is being conducted in 15 regions across the country [24]. As part of this study, we are conducting a follow-up survey of approximately 5,000 children in Amagasaki City, Hyogo Prefecture. Amagasaki is an industrial city located in western Japan and has experienced severe air pollution in the past. Although the concentrations of air pollutants, including PM2.5, have gradually decreased recently, the effects on asthma and allergies, which are common diseases in school-age children, have not been investigated. This study aimed to evaluate asthma and allergies among children participating in the JECS in the second grade of elementary school (8 years old) to clarify associations with individual exposure concentrations to ambient PM2.5 and its chemical components estimated from conception to six years of age.
This was an Adjunct Study of the JECS. The protocol of the JECS and the baseline profiles of the participants have been described in other studies [24, 25]. The JECS is an ongoing nationwide birth cohort study that recruited approximately 100,000 pregnant women across 15 regions in Japan from January 2011 to March 2014. After registration, expecting mothers completed self-administered questionnaires twice during pregnancy: in the first and second/third trimester. Children were followed up primarily through self-administered questionnaires completed by their mothers or guardians 1 month after birth and subsequently every 6 months. The characteristics of the JECS participants were confirmed to be comparable to those of the general Japanese population [25]. The Hyogo Regional Center covers approximately 5,000 participants in Amagasaki City, Hyogo Prefecture. Amagasaki is located in an urban district in western Japan with a land area of 50.7 km2 and a population of approximately 458,000 as of 2023.
This study was conducted as a supplementary survey to the face-to-face examinations conducted by the JECS during the second year of elementary school for participating children. The participants, born between 2011 and 2014, underwent examinations conducted over four years from FY2019 to 2022 when the participants were in the second grade of elementary school. Examination sites were set up at public facilities in the survey region during long vacations or weekends. Children came to the sites with their guardians to undergo physical measurements, urine tests, and mental and neurological developmental assessments. This supplementary survey included a questionnaire survey regarding asthma and allergies and blood sampling.
Detailed instructions for the supplementary survey and a guidance document for the school examination were sent via mail. Those whose written consent was obtained through written and verbal explanations given to their guardians (proxy legal guardians) when they arrived at the examination site were selected as participants. In total, 2,517 children (52.2% of the JECS registrants during this study period) participated in the JECS school examinations. Of these, 2,408 children consented to the Adjunct Study and completed a questionnaire on respiratory/allergic symptoms. Blood samples were collected from 2,326 children. Consequently, 2,408 and 2,326 children were included in the analyses for respiratory/allergic symptoms and allergy examinations, respectively (Fig. 1). This study was approved by the Hyogo Medical University ethical review committee (approval number: 3193; May 14, 2019).

Flowchart of study participant selection.
Abbreviations: JECS, Japan Environment and Children’s Study.
For assessing the respiratory/allergic symptoms of the participants, guardians completed a questionnaire based on the American Thoracic Society, Division of Lung Diseases (ATS-DLD-78-C) [26] and the International Study of Asthma and Allergies in Childhood (ISAAC) [27], widely used internationally. This aimed to evaluate the presence or absence of asthma-related symptoms, wheezing, rhinitis, and rhinoconjunctivitis.
According to the responses to the questionnaire, participants were determined to have a history of asthma based on “yes” answers to the following five questions: “Has your child ever had an attack of wheezing or whistling accompanied dyspnea?” “Has he/she ever had 2 or more such episodes?” “Has he/she been ever diagnosed with asthma by a physician?” “On that occasion, did his/her chest sound wheezy or produce a whistling sound?” and “At that time, did he/she have difficulty in breathing, accompanied by wheezing or whistling?” Wheezing was defined as two or more instances of wheezing or whistling in the chest during the past 2 years. Rhinitis was based on “yes” to the following two questions: “Has your child ever had a problem with sneezing or runny or blocked nose when he/she did not have a cold or the flu?” and “In the past 12 months, has your child had a problem with sneezing, or runny or blocked nose when he/she did not have a cold or the flu?” Rhinoconjunctivitis was defined as rhinitis with itchy-watery eyes in the past 12 months.
Additionally, 5 mL of venous blood was collected from the children, and serum total IgE and specific IgE antibody titers to house dust mite (HDM, Dermatophagoides pteronyssinus) and animal proteins (a mix of Cat dander, Dog dander, Guinea pig epithelium, Rat, and Mouse) were quantified using the ImmunoCAP method (Thermo Fisher Scientific, Inc., Uppsala, Sweden). The detection limits of total IgE and specific IgE were 5 IU/mL and 0.1 UA/mL, respectively. Serum total IgE of 170 IU/mL or more and specific IgE of 0.35 UA/mL or more were considered positive [13, 28].
Exposure assessmentThe concentrations of PM2.5 mass and main chemical component (sulfate, SO42−; nitrate, NO3−; ammonium, NH4+; elemental carbon, EC; organic carbon, OC) during pregnancy and early childhood were estimated daily with a spatial resolution of 1 km × 1 km by daily prediction models of PM2.5 mass and main chemical components from 2010 to 2020, which ranged from the participants’ conception to six years of age.
Details of the model construction have been reported separately [29, 30]. In summary, PM2.5 and its main components were modeled using random forest, a type of machine learning, with various predictors including chemical transport model outputs, meteorological parameters, associated criteria pollutant concentrations, and traffic and land use variables. The model predicted the daily variations well, and Pearson’s correlation coefficients between the predictions and observed values at continuous monitors were 0.75–0.88 for individual components. The residence from conception until six years of age (including relocation information) for each participant was geocoded into a 1 km × 1 km grid consistent with the model prediction grid. Mean PM2.5 mass and main chemical component exposure concentrations were then estimated, considering the date of conception, birth, and relocation (if any) for the first (less than 14 weeks), second (14–27 weeks), and third (28 weeks and beyond) trimesters of pregnancy, as well as 0–1 year, 1–3 years, and 3–6 years after birth. Mean values for the entire pregnancy period and early childhood (0–6 years) were also calculated.
Statistical analysisChildren who participated in this study during the four-year period from FY2019 to 2022 had the results of their respiratory/allergic symptoms and blood examination for allergies, as well as data obtained through questionnaires collected during their mother’s pregnancy and from birth to when they were four years old. The data collected by the JECS to date were utilized to analyze the association between the ambient concentration of PM2.5 mass and each main component estimated using the model constructed for this study.
The prevalence rates of respiratory/allergic symptoms and the results of allergy examinations were compared among fiscal years using a Pearson’s χ2-test. Each outcome was designated as a dependent variable, with the estimated exposure concentrations of the PM2.5 mass and each chemical component from conception to six years of age considered independent variables. A multiple logistic regression analysis was conducted after adjustment for the following covariates: survey year, sex, season of birth, feeding method during infancy, presence of siblings, history of lower respiratory tract infection, use of childcare facilities, passive smoking, presence of pets, mother’s age, history of allergic diseases, smoking during pregnancy, and annual household income. Missing values for covariates were supplemented using multiple imputations to reduce potential non-response bias. The 20 datasets for each imputed variable were created. Results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs) of each outcome per interquartile range (IQR) increase of the estimated concentration of ambient PM2.5 mass and each chemical component. IBM SPSS Statistics version 27 (IBM, Armonk, NY) was used for analysis, and a p-value <0.05 was considered significant.
During the four-year period from FY2019 to 2022, 2,408 children participated in the Adjunct Study, and blood samples were collected from 2,326 children (Fig. 1). Table 1 shows the characteristics of the participants. Though there are differences in the number of participants in each fiscal year due to the time of birth of the participants, consent rates for the participants in each fiscal year were as follows: FY2019, 52.9%; FY2020, 46.1%; FY2021, 51.8%; and FY2022, 52.1%. The consent rate in FY2020 was slightly low due to a period in which examinations could not be conducted amid the spread of Coronavirus Disease 2019 (COVID-19).
| Blood present (n = 2,326) | Blood absent (n = 82) | Overall (n = 2,408) | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Survey fiscal year | ||||||
| FY2019 | 402 | (17.3) | 28 | (34.1) | 430 | (17.9) |
| FY2020 | 786 | (33.8) | 17 | (20.7) | 803 | (33.3) |
| FY2021 | 799 | (34.4) | 25 | (30.5) | 824 | (34.2) |
| FY2022 | 339 | (14.6) | 12 | (14.6) | 351 | (14.6) |
| Sex | ||||||
| Female | 1098 | (47.2) | 38 | (46.3) | 1136 | (47.2) |
| Male | 1228 | (52.8) | 44 | (53.7) | 1272 | (52.8) |
| Season of birth | ||||||
| March–May | 553 | (23.8) | 29 | (35.4) | 582 | (24.2) |
| June–August | 601 | (25.8) | 15 | (18.3) | 616 | (25.6) |
| September–November | 607 | (26.1) | 21 | (25.6) | 628 | (26.1) |
| December–February | 565 | (24.3) | 17 | (20.7) | 582 | (24.2) |
| Feeding method during infancy | ||||||
| Breast milk only | 812 | (34.9) | 26 | (31.7) | 838 | (34.8) |
| Bottle-milk | 163 | (7.0) | 5 | (6.1) | 168 | (7.0) |
| Mixed feeding | 1327 | (57.1) | 51 | (62.2) | 1378 | (57.2) |
| Missing | 24 | (1.0) | 0 | (0.0) | 24 | (1.0) |
| Presence of siblings | ||||||
| No | 1046 | (45.0) | 43 | (52.4) | 1089 | (45.2) |
| Yes | 1256 | (54.0) | 39 | (47.6) | 1295 | (53.8) |
| Missing | 24 | (1.0) | 0 | (0.0) | 24 | (1.0) |
| History of lower respiratory tract infection (up to two years old) | ||||||
| Yes | 235 | (10.1) | 5 | (6.1) | 240 | (10.0) |
| No | 2014 | (86.6) | 73 | (89.0) | 2087 | (86.7) |
| Missing | 77 | (3.3) | 4 | (4.9) | 81 | (3.4) |
| Passive smoking (at three years old) | ||||||
| Yes | 378 | (16.3) | 10 | (12.2) | 388 | (16.1) |
| No | 1835 | (78.9) | 67 | (81.7) | 1902 | (79.0) |
| Missing | 113 | (4.9) | 5 | (6.1) | 118 | (4.9) |
| Pets (three years) | ||||||
| Yes | 360 | (15.5) | 10 | (12.2) | 370 | (15.4) |
| No | 1872 | (80.5) | 67 | (81.7) | 1939 | (80.5) |
| Missing | 94 | (4.0) | 5 | (6.1) | 99 | (4.1) |
| Childcare facility use (at three years old) | ||||||
| Yes | 1188 | (51.1) | 36 | (43.9) | 1224 | (50.8) |
| No | 995 | (42.8) | 39 | (47.6) | 1034 | (42.9) |
| Missing | 143 | (6.1) | 7 | (8.5) | 150 | (6.2) |
| Age of mother (at time of birth) | ||||||
| Mean (SD) | 32.1 | (4.8) | 32.5 | (4.8) | 32.1 | (4.8) |
| <30 | 728 | (31.3) | 21 | (25.6) | 749 | (31.1) |
| ≥30 | 1598 | (68.7) | 61 | (74.4) | 1659 | (68.9) |
| Mother’s history of allergies | ||||||
| Yes | 1202 | (51.7) | 39 | (47.6) | 1241 | (51.5) |
| No | 1117 | (48.0) | 43 | (52.4) | 1160 | (48.2) |
| Missing | 7 | (0.3) | 0 | (0.0) | 7 | (0.3) |
| Mother’s smoking history | ||||||
| Yes | 756 | (32.5) | 32 | (39.0) | 788 | (32.7) |
| (smoking even during pregnancy) | 62 | (2.7) | 4 | (4.9) | 66 | (2.7) |
| No | 1543 | (66.3) | 50 | (61.0) | 1593 | (66.2) |
| Missing | 27 | (1.2) | 0 | (0.0) | 27 | (1.1) |
| Mother’s educational background | ||||||
| ≤12 years | 622 | (26.7) | 22 | (26.8) | 644 | (26.7) |
| ≥13 years | 1690 | (72.7) | 59 | (72.0) | 1749 | (72.6) |
| Missing | 14 | (0.6) | 1 | (1.2) | 15 | (0.6) |
| Household annual income during pregnancy | ||||||
| <4 million JPY | 744 | (32.0) | 24 | (29.3) | 768 | (31.9) |
| ≥4 million JPY | 1497 | (64.4) | 55 | (67.1) | 1552 | (64.5) |
| Missing | 85 | (3.7) | 3 | (3.7) | 88 | (3.7) |
Abbreviations: FY, fiscal year; SD, standard deviation; JPY, Japanese yen.
The annual trend of PM2.5 concentrations at a monitoring station in the study region is shown in Fig. S1. The concentration showed a slight increase in 2013 but thereafter gradually decreased. In 2020, a state of emergency for COVID-19 was declared by the Japanese government, but it had little effect on the annual trend of PM2.5 concentrations.
Table 2 shows the statistics of each estimated concentration of PM2.5 mass and main chemical components estimated for each participant during pregnancy and from birth to six years of age using the model constructed for this study. The mean (standard deviation) of the estimated PM2.5 concentration was 14.4 (1.2) µg/m3 during the entire pregnancy period and 13.1 (0.8) µg/m3 after birth, with the postnatal value being slightly lower. As shown in Table S1, the estimated concentrations of PM2.5 mass and main chemical components during pregnancy and early childhood were high for all components during pregnancy, gradually decreasing after birth.
| Mean | SD | Min | Percentiles | Max | IQR | |||
|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||||
| Entire pregnancy (n = 2,380) | ||||||||
| PM2.5 | 14.4 | 1.2 | 11.0 | 13.5 | 14.3 | 15.2 | 18.2 | 1.6 |
| SO42− | 4.06 | 0.42 | 2.81 | 3.74 | 4.04 | 4.34 | 5.37 | 0.60 |
| NO3− | 1.05 | 0.22 | 0.39 | 0.88 | 1.06 | 1.22 | 1.63 | 0.34 |
| NH4+ | 1.75 | 0.17 | 1.24 | 1.62 | 1.76 | 1.87 | 2.29 | 0.25 |
| EC | 1.00 | 0.08 | 0.81 | 0.94 | 0.99 | 1.05 | 1.29 | 0.11 |
| OC | 3.29 | 0.15 | 2.73 | 3.18 | 3.29 | 3.40 | 3.73 | 0.22 |
| First 6 years after birth (n = 2,352) | ||||||||
| PM2.5 | 13.1 | 0.8 | 10.7 | 12.6 | 13.1 | 13.6 | 15.9 | 1.0 |
| SO42− | 3.62 | 0.20 | 3.11 | 3.48 | 3.64 | 3.76 | 4.22 | 0.28 |
| NO3− | 0.94 | 0.10 | 0.57 | 0.86 | 0.93 | 0.99 | 1.32 | 0.13 |
| NH4+ | 1.55 | 0.09 | 1.28 | 1.49 | 1.56 | 1.62 | 1.86 | 0.13 |
| EC | 0.90 | 0.07 | 0.69 | 0.85 | 0.89 | 0.95 | 1.22 | 0.10 |
| OC | 3.15 | 0.11 | 2.59 | 3.08 | 3.15 | 3.23 | 3.53 | 0.15 |
Abbreviations: SD, standard deviation; IQR, interquartile range; PM2.5, particulate matter with a diameter of 2.5 µm or less; SO42−, sulfate; NO3−, nitrate; NH4+, ammonium; EC, elemental carbon; OC, organic carbon.
The prevalence rates of asthma and wheezing were highest in FY2019 among both males and females and lowest in FY2022 for males and in FY2021 among females, though the difference was not significant. There were no differences in the prevalence of rhinitis and rhinoconjunctivitis between fiscal years (Table 3).
| Respiratory/allergic symptom prevalence | Blood test for allergies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Asthma (%) |
Wheezing (%) |
Rhinitis (%) |
Rhinoconjunctivitis (%) |
n | Total IgE ≥ 170 IU/mL (%) |
Sensitization to HDM (%) |
Sensitization to Animal proteins (%) |
|
| Male | |||||||||
| 2019 | 222 | 5.0 | 12.0 | 49.1 | 11.2 | 209 | 44.0 | 60.8 | 30.1 |
| 2020 | 422 | 5.0 | 8.6 | 51.2 | 10.5 | 412 | 49.8 | 67.0 | 36.4 |
| 2021 | 426 | 4.7 | 8.5 | 48.3 | 10.6 | 414 | 48.3 | 64.3 | 35.7 |
| 2022 | 202 | 4.3 | 6.4 | 53.3 | 12.4 | 193 | 56.0 | 71.5 | 35.9 |
| Four years | 1272 | 4.8 | 8.8 | 50.2 | 11.0 | 1228 | 49.8 | 65.7 | 35.0 |
| p-value† | 0.982 | 0.251 | 0.672 | 0.893 | 0.113 | 0.118 | 0.432 | ||
| Female | |||||||||
| 2019 | 208 | 5.3 | 8.7 | 33.3 | 6.7 | 193 | 32.1 | 48.2 | 24.5 |
| 2020 | 381 | 2.9 | 6.1 | 38.8 | 9.2 | 374 | 41.7 | 54.8 | 28.3 |
| 2021 | 398 | 1.5 | 4.5 | 36.2 | 7.3 | 385 | 40.5 | 52.3 | 29.9 |
| 2022 | 149 | 2.2 | 6.7 | 38.1 | 8.7 | 146 | 37.0 | 52.7 | 17.8 |
| Four years | 1136 | 2.8 | 6.1 | 36.8 | 8.0 | 1098 | 39.0 | 52.5 | 26.8 |
| p-value† | 0.075 | 0.252 | 0.591 | 0.672 | 0.127 | 0.523 | 0.024 | ||
| Both sexes | |||||||||
| 2019 | 430 | 5.1 | 10.4 | 41.5 | 9.0 | 402 | 38.3 | 54.7 | 27.4 |
| 2020 | 803 | 4.0 | 7.4 | 45.3 | 9.9 | 786 | 45.9 | 61.2 | 32.6 |
| 2021 | 824 | 3.2 | 6.6 | 42.5 | 9.0 | 799 | 44.6 | 58.5 | 33.0 |
| 2022 | 351 | 3.4 | 6.6 | 46.9 | 10.9 | 339 | 47.8 | 63.4 | 28.1 |
| Four years | 2408 | 3.8 | 7.5 | 43.9 | 9.6 | 2326 | 44.4 | 59.5 | 31.2 |
| p-value† | 0.380 | 0.107 | 0.328 | 0.768 | 0.038 | 0.066 | 0.108 | ||
†Pearson’s χ2 test for comparison among fiscal years. Bold, p < 0.05.
The percentage of serum total IgE ≥ 170 IU/mL was 49.8% for males and 39.0% for females, and the percentages of sensitizations to HDM and animal proteins were also higher among males than females. The percentages of elevated total IgE levels and sensitization to HDM were highest in FY2022 among males and FY2020 among females, though the difference was not significant. The percentage of sensitization to animal proteins in FY2022 was significantly lower among females (Table 3).
Relationship of estimated concentrations of ambient PM2.5 mass and chemical components during pregnancy and early childhood with respiratory/allergic symptomsFigure 2 shows the results of a multiple logistic regression analysis of the relationship of estimated concentrations of ambient PM2.5 mass and chemical components by period with respiratory/allergic symptoms. Detailed ORs and 95% CIs are presented in Table S2.

Relationship of PM2.5 chemical components during pregnancy and early childhood with respiratory/allergic symptoms.
Data are shown as odds ratios and 95% confidence intervals for each outcome, associated with per interquartile range increase in each of PM2.5 mass and main chemical components, after adjustment for the covariates. Abbreviations: PM2.5, particulate matter with a diameter of 2.5 µm or less; SO42−, sulfate; NO3−, nitrate; NH4+, ammonium; EC, elemental carbon; OC, organic carbon; T1, first trimester; T2, second trimester; T3, third trimester; Entire Preg, entire pregnancy; yo, years old.
Asthma exhibited the largest OR per IQR increase in estimated SO42− concentration during the first trimester of pregnancy at 1.40 (95% CI: 0.77, 2.54); however, this was not significant. No significant associations were observed with the estimated concentrations of other components in any period. Wheezing was significantly associated with estimated NO3− and EC concentrations throughout the pregnancy period, with ORs of 1.64 (95% CI: 1.10, 2.47) and 1.38 (95% CI: 1.07, 1.78) per IQR increase, respectively. Additionally, a significant association was found with estimated PM2.5, EC, and OC concentrations in the second trimester and estimated NO3−, EC, and OC concentrations in 1–3 years after birth. Rhinitis exhibited a significant association with estimated PM2.5 concentrations during the second trimester, with an OR of 1.50 (95% CI: 1.15, 1.95) per IQR increase. Furthermore, there was a significant association with estimated NO3− concentrations in the first trimester and estimated SO42− and NH4+ concentrations in the second trimester. Rhinoconjunctivitis displayed a significant association with estimated SO42− concentrations during the second trimester, with an OR of 1.46 (95% CI: 1.01, 2.13) per IQR increase. Meanwhile, rhinoconjunctivitis was negatively associated with estimated PM2.5 and OC concentrations in the third trimester, with ORs of 0.60 (95% CI: 0.39, 0.91) and 0.65 (95% CI: 0.50, 0.86) per IQR increase, respectively.
Relationship of estimated concentrations of ambient PM2.5 mass and chemical components during pregnancy and early childhood with allergy examination resultsFigure 3 shows the results of a multiple logistic regression analysis of the relationship of estimated concentrations of ambient PM2.5 mass and chemical components for each period with allergy examination results. Detailed ORs and 95% CIs are presented in Table S3.

Relationship of PM2.5 chemical components during pregnancy and early childhood with allergy examination results.
Data are shown as odds ratios and 95% confidence intervals for each outcome, associated with per interquartile range increase in each of PM2.5 mass and main chemical components, after adjustment for the covariates. Abbreviations: PM2.5, particulate matter with a diameter of 2.5 µm or less; SO42−, sulfate; NO3−, nitrate; NH4+, ammonium; EC, elemental carbon; OC, organic carbon; T1, first trimester; T2, second trimester; T3, third trimester; Entire Preg, entire pregnancy; yo, years old.
High serum total IgE levels were significantly associated with estimated SO42− concentrations throughout pregnancy and in estimated NH4+ concentrations in the first trimester of pregnancy, with ORs of 1.21 (95% CI: 1.00, 1.46) and 1.26 (95% CI: 1.01, 1.58) per IQR increase, respectively. Conversely, higher estimated PM2.5, SO42−, NH4+, and EC concentrations were found to be negatively associated at various periods after birth. Sensitization to HDM was significantly associated with estimated PM2.5, SO42−, and NH4+ concentrations throughout the pregnancy period, with ORs of 1.26 (95% CI: 1.05, 1.51), 1.38 (95% CI: 1.14, 1.57), and 1.41 (95% CI: 1.15, 1.74) per IQR increase, respectively. During the first trimester, higher estimated exposures to all components other than SO42− were significantly associated with sensitization to HDM. However, the association with estimated SO42− concentration in 1–3 years after birth was significantly negative, with an OR of 0.83 (95% CI: 0.69, 0.99). Sensitization to animal proteins was significantly associated with estimated PM2.5 concentrations in the first trimester of pregnancy, with an OR of 1.44 (95% CI: 1.13, 1.82) per IQR increase. Significant associations were also observed with estimated SO42−, NH4+, EC, and OC concentrations during the same period. However, no associations with concentration were observed in any other period.
This birth cohort study analyzed the association between the results of respiratory/allergic symptoms and blood examination for allergies in the second grade of elementary school (eight years old), and estimated concentrations of PM2.5 mass and main chemical component for each participant in each period during pregnancy and early childhood. Asthma did not show a significant association with estimated exposure in any period. However, estimated concentrations of PM2.5 during the second trimester of pregnancy and NO3−, EC, and OC throughout pregnancy and 1–3 years after birth were associated with a significant increase in wheezing. Furthermore, higher estimated concentrations of PM2.5, SO4−, and NH4+ during the second trimester of pregnancy were associated with a higher risk of rhinitis. For allergic status, estimated exposure to PM2.5 and several components during pregnancy was associated with elevated serum total IgE levels and sensitization to HDM and animal proteins. However, there was no association with postnatal exposure.
Numerous previous reports have indicated that exposure to air pollutants such as PM2.5 during pregnancy and early childhood was a risk factor for the onset of asthma, wheezing, rhinitis, and allergen sensitization in childhood [2, 10–15, 21]. However, a cohort study in Sweden found no association between PM2.5 exposure during pregnancy and one year after birth and asthma, although PM2.5 exposure during the first three years of life was a risk factor for asthma onset [31]. A systematic review of the association of air pollution during pregnancy with asthma and wheezing in children indicated that exposure to nitrogen dioxide (NO2) and sulfur dioxide (SO2) was a clear risk factor but that there was insufficient evidence for exposure to PM2.5 and BC [32]. However, some European birth cohort studies showed an association between postnatal PM2.5 concentration and asthma onset after the age of four years when following up to the age of 14–16 years [6]. These results show the lack of consensus on the effects of prenatal and postnatal PM2.5 exposure on asthma and wheezing onset in children. In the present study, there was no association between estimated exposure to PM2.5 and its main chemical components during pregnancy and childhood and asthma at eight years of age. However, elevated NO3−, EC, and OC exposure concentrations during pregnancy and up to three years after birth were associated with a significant increase in the prevalence of wheezing. These findings align with the results of some previous studies [11, 19, 20] conducted in countries, where air pollution levels were considerably low. Since NO3− is generated secondarily from nitrogen oxides emitted mainly by automobiles and EC is predominantly emitted by diesel automobiles [33], it is suggested that traffic-related air pollution exerts a substantial impact.
A systematic review examining the relationship between air pollution and rhinitis indicated that children were more susceptible to air pollutants including PM2.5 [15, 34]. However, this association was shown to vary by country and region, with the effect being more pronounced in developing countries in Asia, where air pollution levels are high [14]. Regarding allergen sensitization, several studies reported an association between exposure to PM2.5 and BC and sensitization to airborne and food allergens [15, 35]. Notably, no such association was observed in a study conducted in Norway, possibly due to its low air pollution levels [36]. A meta-analysis of five European birth cohort studies did not find an association between postnatal exposure to air pollution and rhinoconjunctivitis/allergen sensitization in children aged 4–8 years [16, 17]. Of these five studies, four reported that air pollution exposure did not increase the risk of rhinoconjunctivitis even when following up until the age of 16 years [6]; however, an association with sensitization to grass pollen and cat allergen was observed [37]. In China, a study reported an association between NO2 exposure during pregnancy and one year after birth and allergic diseases such as hay fever and rhinitis in children aged 4–6 years, although PM2.5 was not specifically investigated [38]. Meanwhile, a birth cohort study in Canada found that food and inhaled allergen sensitization at one year of age was associated with traffic-related air pollution exposure one year after birth but was not associated with air pollution during pregnancy [39]. Another study demonstrated that diesel exhaust particle exposure increased airborne allergen sensitization at 2–3 years of age and was a risk factor for rhinitis onset at four years of age [40]. Additionally, it was reported that the oxidative potential of PM2.5 was associated with rhinitis onset but not with sensitization [41]. In this study, exposure to PM2.5, SO42−, NO3−, and NH4+ during pregnancy was associated with an increased prevalence of rhinitis and rhinoconjunctivitis, although the concentrations were relatively low. Additionally, this study also revealed that exposure to PM2.5, SO42−, and NH4+, which are considered to be influenced by long-range transport from the Asian continent [30], during pregnancy was associated with elevated serum total IgE levels as well as sensitization to HDM and animal proteins. Conversely, an opposite association was partially observed with postnatal exposure; therefore, this mechanism requires further investigation.
Ambient PM2.5 is generated from diverse natural and anthropogenic sources, with composition varying across countries and regions [18, 42, 43]. Primary components in particles, such as OC and BC, are released directly into the atmosphere from sources. In contrast, pollutants such as SO42−, NH4+, and NO3− are generated secondarily through chemical reactions in the atmosphere [33]. The association between the chemical components in PM2.5 and respiratory diseases or allergen sensitization in children has been examined [5]. A birth cohort study in the United States reported an association between fetal exposure to ambient NO3− and asthma onset in six-year-old children [19]. Similarly, a retrospective cohort study in Canada demonstrated an association between exposure to PM2.5, BC, NH4+, NO3−, and organic matter during pregnancy and early childhood and asthma onset up to six years of age [20]. In China, where PM2.5 concentrations are significantly higher than in the United States and Canada, an association was observed between PM2.5 exposure during pregnancy and early childhood and asthma/wheezing onset up to six years of age, particularly combustion-derived BC, organic matter, and SO42− [21]. A recently reported cohort study of singleton births in Denmark revealed that PM2.5 exposure during pregnancy and childhood was associated with asthma onset up to 19 years of age. This study also identified associations with EC, OC, SO42−, and NH4+ in PM2.5 with OC, derived mainly from biomass combustion, showing a particularly substantial influence [11]. In low- and middle-income countries in Asia, Africa, and Central/South America, the effects of exposure to household PM2.5 from cooking and heating on adverse perinatal outcomes and reduced lung function among children [44–46].
Many unknown aspects exist regarding the biological mechanisms by which PM contributes to the onset of asthma in children. PM2.5 can permeate the maternal alveoli and placental barrier during the fetal period [8]. It has also been suggested that PM may induce systemic inflammation in pregnant mothers, reducing nutrient and oxygen supply to the fetus and indirectly affecting fetal lung function [8]. After birth, environmental factors induce oxidative stress and damage in children, known to result in airway wall remodeling, initiation of inflammatory pathways and immunological effects, and enhanced respiratory sensitization to allergens [47, 48].
It has been proposed that the effects of air pollutants on rhinitis onset are mainly due to oxidative stress and inflammatory responses in human nasal epithelial cells [49–51]. Exposure to air pollutants induces allergic immune responses, including changes in serum total IgE levels in mice [52]. Air pollutants can also adhere to pollen and induce allergies in humans sensitized to pollen [53]. These studies collectively suggest that air pollution may influence allergic diseases through immune responses.
This study has some limitations. First, this study exclusively included participants covered by the Hyogo Regional Center of the JECS, implying that most participants resided in a single city. While ambient PM2.5 concentrations varied within the city, the differences were fewer when compared with those across Japan, rendering it insufficient for adequately detecting the influence of exposure. Second, this study focused solely on analyzing exposure to ambient PM2.5 and its main components; other air pollutants were not considered. Notably, NO2 and SO2 concentrations in the target region were very low. Ozone (O3) concentrations were relatively high with significant seasonal variations; however, they were not considered in this study due to their widespread presence and minimal concentration differences within the target region. The JECS is being implemented in 15 regions in Japan, and it is anticipated that air pollutant exposure across Japan will be evaluated and its effects clarified in the future. Third, the concentrations of PM2.5 mass, carbons, and main ionic components were estimated in this study; however, elemental components were not considered. Although the proportion of elemental components in PM2.5 is smaller than that of carbons and ionic components, associations have been observed between elemental components such as potassium and sulfur in PM2.5 and asthma and rhinitis [5, 6]. Future considerations should involve examining the relationship with elemental components. Fourth, outdoor PM2.5 exposure concentration was estimated in this study; however, indoor exposure was not considered. Given that children spend extended periods indoors, the effects of indoor air pollution must also be considered [54, 55]. The JECS involves measurements of indoor air pollutants for some participants at 1.5 and 3 years old, and these results need to be utilized for indoor air pollution evaluations. Fifth, exposure to PM2.5 mass and five chemical components was estimated in different periods during pregnancy and early childhood, and the association with outcomes was analyzed, raising the possibility of repeated significance testing. We did not counter the potential problem, but we believe that the results may be important per the precautionary principle. Lastly, only outcomes at eight years old were considered in this study. There are plans in the JECS to follow up on participants, and it is anticipated that the long-term effects of air pollution on allergic disease onset or allergen sensitization will be revealed.
Despite the limitations, there are several strengths in this study. First, as the participants are those from a birth cohort study, the children’s place of residence could be precisely determined daily from conception to the age of six years, allowing for an accurate evaluation of the concentrations of PM2.5 and the main component for each period using an exposure concentration estimation model. Second, regular follow-up surveys from pregnancy to the present day were conducted, providing sufficient information on the past medical history, living environment, and socioeconomic status of the mother and child for appropriate adjustments during analysis. Third, guardians were interviewed face-to-face when the child was eight years old, and blood samples were collected to objectively assess the state of allergen sensitization, facilitating an analysis of the relationship with PM2.5 exposure both before and after birth.
The prevalence rate of asthma was slightly lower and the percentage of sensitization to HDM was higher than those of the previous study conducted in this region [28], although the study methods were different. Recently, the concentrations of air pollutants, including PM2.5, are gradually decreasing [29, 30]. In this study, no association between asthma and exposure to PM2.5 and its main components may be due to low levels of air pollution in this region. However, we could observe the associations of PM2.5 and its main components with wheezing, rhinitis, and allergen sensitization among children. Therefore, the findings of this study can be generalized to various regions with low concentrations of air pollution.
The associations were observed between estimated exposure to NO3−, EC, and OC during pregnancy and early childhood and an increased incidence of wheezing in children at eight years of age. Additionally, increased incidence of rhinitis due to PM2.5, SO4−, and NH4+ exposure during pregnancy was observed. However, there was no association between asthma and exposure to PM2.5 and its main components at any period. Regarding allergen sensitization, an association was observed between SO42− and NH4+ exposure during pregnancy and sensitization to HDM and animal proteins; however, no association was observed with postnatal exposure. The components identified as being associated with allergic symptoms and sensitization in this study were primarily derived from automobiles and biomass combustion. The results emphasize the importance of addressing air pollution to mitigate the risk of wheezing, asthma, and allergies in school-age children.
ammonium
ATS-DLDAmerican Thoracic Society, Division of Lung Diseases
CIconfidence interval
COVID-19Coronavirus Disease 2019
ECelemental carbon
HDMhouse dust mite
ISAACInternational Study of Asthma and Allergies in Childhood
IQRinterquartile range
JECSJapan Environmental and Children’s Study
NO3−nitrate
NO2nitrogen dioxide
OCorganic carbon
ORodds ratio
O3ozone
PM2.5particulate matter with a diameter of 2.5 µm or less
SO42−sulfate
SO2sulfur dioxide
WHOWorld Health Organization
This study design was approved by the Ethics Review Board of the Hyogo Medical University (Approval number: 3193). This study was conducted in accordance with the Declaration of Helsinki and other internationally recognized regulations. Written informed consent was obtained from all caregivers.
Consent for publicationNot applicable.
Availability of data and materialsData will be made available upon reasonable request.
Competing interestThe authors declare that they have no competing interests.
FundingThis work was supported by JSPS KAKENHI [Grant Numbers JP18H03060, JP21H03205] and the Environment Research and Technology Development Fund [JPMEERF20185002, JPMEERF20195055, JPMEERF20215005] provided by the Environmental Restoration and Conservation Agency of Japan. The JECS was funded by the Ministry of the Environment, Japan.
Authors’ contributionKO: methodology, investigation, data curation, formal analysis, writing - original draft preparation; YY: methodology, investigation, data curation, formal analysis, visualization; SA: methodology, software, investigation, data curation; HS: methodology, software, investigation, supervision, funding acquisition; NT: methodology, investigation, data curation; YT: conceptualization, methodology, investigation; MS: conceptualization, methodology, investigation, supervision, project administration, writing- reviewing and editing, funding acquisition. All authors reviewed and approved the final manuscript.
AcknowledgmentsThis study was conducted as an Adjunct Study of the Japan Environment and Children’s Study (JECS). We would like to express our gratitude to all participants and research staff at the Hyogo Regional Center. The JECS was funded by the Ministry of the Environment, Japan. The conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.