2021 Volume 1 Pages 91-96
2021 Volume 1 Pages 91-96
Heavy metal ions greatly impact the physiological activities of aquatic plants. Effects of the ions Cu2+, Ni2+, and Co2+ on the physiological activities of aquatic plant were studied using the dissolved oxygen (DO)-quenched fluorescence/materials movement-induced probe beam deflection method. An Ru(II) complex was used as a fluorescence probe, with Egeria densa as the model aquatic plant for this study. DO-quenched fluorescence of the Ru(II) complex as well as the materials movement-induced beam deflection at vicinities of the aquatic plant were simultaneously monitored for real-time observations. The simulataneous monitoring were conducted on various plant parts with and without the presence of 1-μM heavy metal ions, such as Cu2+, Ni2+, and Co2+. Results showed that the presence of the 1-μM heavy metal ions greatly altered changing trends of both DO and beam deflection over time in both the photosynthetic and respiration processes for the plant. This altering suggests that the existence of the 1-μM heavy metal ions has greatly changed the physiological processes in the aquatic plant. Based on study results, this method has the potential to be used as a new sensitive tool for monitoring heavy metal stress effects on aquatic plants.
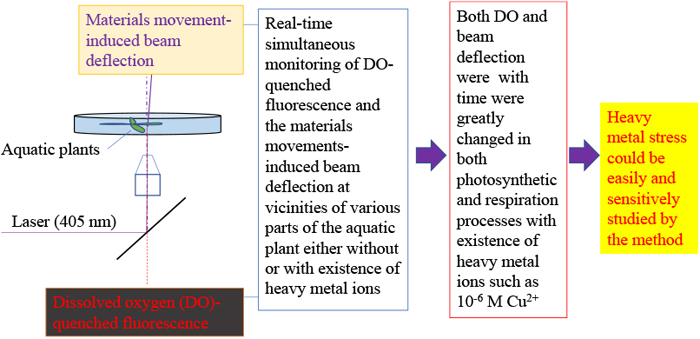
It is well known that plants provide humans with numerous necessities for life through one of their fundamental properties, photosynthesis, including breathable air and food. Physiological activities of plants—such as photosynthesis—are greatly affected by their surrounding environment and any changes within it (Lawlor, 1995). The vital life services that plants provide humans have not been able to protect plants from the negative impact of human-introduced environmental dangers. One of these dangers is the introduction of excessive heavy metal ions into water and soil environments. These are particularly noticeable because heavy metal ions not only hinder crop growth but also in turn pose serious threats to human health through crop contamination (Zhang et al., 2018; Ghori et al., 2019). This vicious cycle has highlighted the importance of studying the effects of heavy metal ions on the physiological activities of plants as well as the effect of the stresses caused by the presence of heavy metal ions.
Conventional methods for studying the stresses caused by heavy metal ions on plants are based on: i) plant growth observation; ii) field sampling and subsequent laboratory chemical analyses; and iii) measurement of concentration changes in either or both CO2 and O2 in plant physiological activities with and without heavy metal ions (Guanzon et al., 1994; Gupta et al., 2013; Zhang et al., 2018). Observing plant growth is generally a time-consuming process conducted over many months. Field sampling and chemical analyses requires complex and expensive tools and is labor heavy. Measuring changes in either or both CO2 and O2 concentrations in plant physiological activities is usually performed in either a closed or open vessel containing a sample plant (Hunt, 2003). Although CO2 and O2 concentrations in the vessel can be measured accurately and sensitively, results are usually spatially and temporally averaged, reflecting the average concentration of CO2/O2 released or absorbed by the whole—or whole parts—of the plant in the vessel. The monitored CO2/O2 does not reflect the real time CO2/O2 movements across the plant surface. This is because it takes time to diffuse or deliver released/absorbed CO2/O2 from the plant surface to the sensor or sampling point in the vessel. Additionally, it is difficult to distinguish either or both CO2 and O2 released or absorbed from different parts of the plant—e.g., leave, stems, and roots.
Recently developed dissolved oxygen (DO)-quenched fluorescence/material movement-induced beam deflection method has presented a novel opportunity to conduct real time monitoring of movement of physiologically active materials across the plant surface (Wu et al., 2017; Wu and Huang, 2018). In the method, a 405-nm probe laser was focused on the near vicinity of an aquatic plant in a cultured solution containing 10−6 M fluorescent ruthenium (II) complex (Ru(II) complex). Fluorescence of the Ru(II) complex at the vicinity was excited; and consequently quenched by DO. A change of DO in plant vicinity could be monitored in situ in real time by analyzing the quenched fluorescence intensity. Meanwhile, deflection of the probe beam, which was generated by the movement of physiologically active materials such as O2 and CO2, thereby inducing concentration gradients, was also monitored in real time. This method enables a more sensitive approach, allowing real time monitoring of DO and material movements in situ within micrometers of the plant surface. Conventional methods are only capable of measuring the average spatial and temporal change in either or both CO2 and O2. Furthermore, the method allows for distinguishing movement of materials such as O2 across different organ surfaces of the of the plant.
In this study, this method was used to examine the effect of heavy metal stress in aquatic plants. Because of the higher sensitivity that this approach has over conventional methods, stress caused by heavy metal ions at a low concentration level is also expected to be revealed. Changes of both DO and materials movement-induced deflection at different organ positions on the plant—such as the leaf, root, and stem—are monitored firstly without heavy metal presence and with the presence of 1-μM heavy metal ions, specifically Cu2+, Ni2+, and Co2+. The 1-μM concentration of heavy metal ions in this study is lower than that recorded in previous research, which generally focused in the 1–1,000 ppm or several 10 μM–several mM ranges (Bazzaz et al, 1974; Clijsters and Assche, 1985; Soltan and Rashed, 2003). Egeria densa Planch species was used as a model aquatic plant. It successfully demonstrated that heavy metal ions of Cu2+, Ni2+, and Co2+ have significant effects on the physiological processes of aquatic plants even with a concentration as lower as 1-μM. It also showed that effects of the heavy metal ions on movement of physiologically active materials such as O2 across surface of the different organs can be distinguishably studied by the method.
The experimental setup for monitoring DO and beam deflection was the same as reported in previous studies (Wu and Huang, 2018). Fig. 1–A illustrates the optical system in the setup. A 405-nm semiconductor laser probe beam (output power: 3.0 mW, Sigma Koki, Japan) was used as the light source for both probe beam and fluorescence measurement. The laser probe beam was reflected off of a dichromic mirror and was focused on near vicinity of different plant organs (Fig. 1–B). The spot size of the beam was estimated to be approximately a few μm. The monitored plant, with a length of about 3 cm, was prepared in a culture dish (φ56 mm×15 mm) containing 20 mL of a 1-μMRu(II) complex solution either with or without 1-μM heavy metal ions, namely Cu2+, Ni2+, or Co2+. The culture dish was then placed on an X-Y-Z micro-stage (Edmund Optics) to account for adjusting distance between the focus point of the probe beam and the plant surface. A piece of slide glass was used to protect the plants to prevent additional and unnecessary movement. Deflection of the laser probe beam was detected by a position sensor consisting of a bi-cell photodiode. Fluorescence transmitted back through the dichromic mirror was monitored by a photomultiplier tube. A commercial DO/temperature sensor (pyro science GmbH) was inserted into the culture dish to monitor DO and temperature change of the bulk culture solution. The monitored temperature, DO, deflection, and fluorescence signals were concurrently input into a digital multimeter (Texio Technology Corporation, Japan) and recorded on a computer. The experimental data were input and analyzed using Excel.

Illustration of the experimental setup (A) and location(s) where the probe beam passed through at various parts of the aquatic plant.
The optical detection system was placed in a dark room with a window through which a red-blue light-emitting diode (LED; output power: 8 W; Luxour, Japan) with wavelengths of ~660 nm and ~460 nm illuminated the aquatic plants during the photosynthetic process. During respiration, the red-blue LED was switched off, with the window of the dark room closed.
Stock solutions of 100-μM Ru (II) complex, Cu2+, Ni2+, and Co2+ were prepared by dissolving certain amounts of the Ru (II) complex (Tris (2, 2’-bipyridyl) ruthenium (II) chloride), CuSO4·5H2O, NiCl2, and CoCl2 in 100 ml of water, respectively. The stock solutions were diluted 100-fold with distilled deionized water for all experiments. All chemicals were analytical grade, received from Wako Chemicals without further purification.
For experimental procedures, 20 mL of 1-μM Ru (II) complex solution either with or without 1-μM heavy metal ion of Cu2+, Ni2+, or Co2+ was firstly added into the culture dish with the aquatic plant. After 30 min, the culture dish solution settled. After this, the probe beam was focused to within a vicinity of 0 μm from the different locations on the aquatic plant by adjusting the X-Y-Z micro-stage. Then, the red-blue LED was either turned ON or OFF, and monitoring processes of the deflection, fluorescence, DO, and temperature (by the commercial DO/temperature sensor) began. After a 2-h monitoring period, observed and captured data were saved on the computer, and another round of monitoring was started.
Similar to previous studies and reports (Wu et al., 2009), the movement of materials across the plant surface generated concentration gradients at vicinities of the plant surface. Additionally, reaction heat of either or both chemical and biochemical processes occurred in the monitored plants induce temperature gradients at the vicinities. Both the concentration and temperature gradients generated refractive index gradients. When a laser probe beam passed through the target points on the plant surface, it deflected by the refractive index gradients. In most cases where no large reaction heat was produced, the temperature gradient-induced deflection could be ignored when compared with the concentration gradient-induced deflection. Considering this, deflection of the probe beam can be considered as generated by the concentration gradients; the deflection angle θ of the probe beam was therefore approximated as follows (Wu, et al., 2009):
| (1) |
Where k is a constant determined by optical path-length and refractive index n in the vicinity of the plant surface; dn/dx is refractive index gradient; dn/dCi is the concentration coefficient of n for chemical species i; dCi/dx is the concentration gradient of i; and m is the number of chemical species present.
Equation (1) shows that deflection of the probe beam is a sum of the ones induced by concentration gradients of the individual components involved in material movements. In respiration and photosynthesis, the main movement of materials takes place with CO2 and O2 across the plant surface. Based on this consideration, the deflection angle could be approximated as:
| (2) |
Where dn/dCO2 and dn/dCCO2 are concentration coefficients of a refractive index for O2 and CO2, respectively; dCO2/dx and dCCO2/dx are concentration gradients of O2 and CO2, respectively. Directions of both the dCO2/dx and dCCO2/dx in respiration are contradictory to those in photosynthesis. Therefore, deflection signals in respiration are expected to be reversed when compared with those in photosynthesis.
Alternately, quenching fluorescence of the Ru (II) complex by DO is described by the Stern–Volmer equation (Ware, 1962):
| (3) |
Where F0 and F are fluorescence intensities of the Ru (II) complex solution without and with DO, respectively; KSV is the Stern–Volmer constant, and CDO is the DO concentration. This equation can be transformed as follows:
| (4) |
As shown in Equation (4), CDO at vicinities on the aquatic plant can be calculated from F0 and F if KSV is known beforehand. On the other hand, because F0, F, and KSV are affected by temperature, effects of temperature change in the experiment must be corrected. Algorithms for obtaining KSV by considering effects of temperature changes have been previously reported (Huang and Wu, 2017; Wu et al, 2017; Wu and Huang, 2018).
Fig. 2 shows the changes in beam deflection (A and B) and DO concentration (C and D) with time during the respiration (A and C: LED OFF), and photosynthetic (B and D: LED ON) processes at different parts on the aquatic plant in a 1-μM Ru (II) complex solution. As shown in Figs. 2–A and 2–B, the change in deflection signal with time was the largest in the middle vicinity and second largest at the apex of a leaf. Changes of deflection signals were small in the leaf base, root, and stem. This suggests that the largest changes of the dCO2/dx and dCCO2/dx occurred at the middle vicinity and the second at the apex of the leaf. Changes of the dCO2/dx and dCCO2/dx at the leaf base, root, and stem were small. This infers that the largest material movements occurred with CO2 and O2 in the middle vicinity, with the second largest at the leaf apex; and only small movements at the leaf base, stem, and root in both respiration and photosynthesis. Reversed changing trends of the deflection signals with time in the respiration and photosynthetic processes were caused by a reversal of both the dCO2/dx and dCCO2/dx, as stated above.
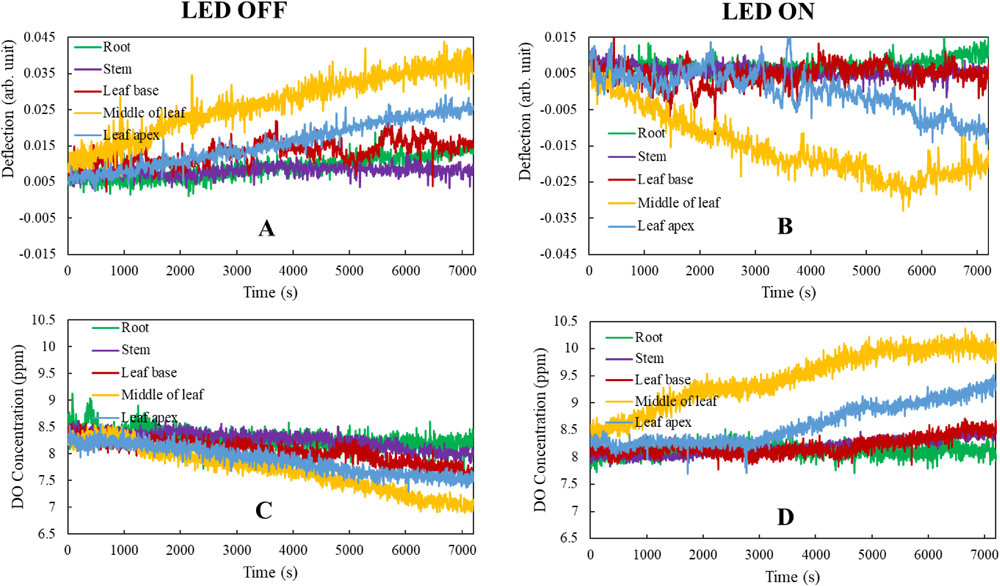
Beam deflection (A and B) and DO concentration (C and D) during respiration (A and C: LED OFF) and photosynthesis (B and D: LED ON) at different points on the plant cultured in a 1-μM Ru (II) complex solution.
Figs. 2–C and 2–D showed DO changes with time during the respiration and photosynthetic processes, respectively. In the middle vicinity, DO decreased from ~8.3 ppm to 5.8 ppm over 2 h during respiration, whereas it increased from ~8.3 ppm to 9.8 ppm during photosynthesis. DO changes were the second largest at the leaf apex in both photosynthesis and respiration. For the leaf base, root, and stem, although changes were much smaller than those in the middle vicinity, DO still increased during photosynthesis while decreasing slightly over time during respiration. Because the middle of the leaf has much more chlorophyll and stomata than other parts, O2 movement was the largest. These results illustrate that this method can distinguish O2 movement across different organ surfaces of the aquatic plant, while conventional methods can only obtain the average in O2 changes.
In view of monitoring and measurement of physiological activities in aquatic plants, the results shown in Fig. 2 highlight that the method is the most sensitive in capturing changes of DO and beam deflection by passing the laser probe beam through middle vicinity of the plant leaf. As previously outlined, conventional methods monitor or measure averages of CO2 and O2 changes within the study container or vessel holding the sample plant. This restriction means that results and subsequent data is much less sensitive than those obtained at the middle vicinity of the plant leaf. In addition, this method is conducted in much more real time than the conventional ones because it involves direct monitoring at the vicinities of the plant surface (Wu et al., 2017; Wu and Huang, 2018).
Fig. 3 shows beam deflection (A and B) and DO concentrations (C and D) at different parts of the plant in a 1-μM Ru (II) complex solution containing 1-μM Cu2+. The largest changes in both beam deflection signals and DO concentrations were observed at the middle of the leaf, with the second largest at the leaf apex in both respiration and photosynthesis. However, changing trends of both beam deflection and DO during photosynthesis (B and D) became comparable to those observed during respiration (A and C). This observation is completely novel when compared with the results illustrated in Figs. 2–B and 2–D, highlighting that the direction of materials movement across the plant surface in different organs during photosynthesis had been completely altered in the presence of just 1-μM Cu2+.

Beam deflection (A and B) and DO concentration (C and D) during respiration (A and C: LED OFF) and photosynthesis (B and D: LED ON) at different points on the plant cultured in a 1-μM Ru (II) complex solution containing 1-μM Cu2+.
As shown in Fig. 3–C, DO concentration decreased from 8.5 ppm to 1.5 ppm, 8.7 ppm to 4.0 ppm, 8.5 ppm to 6.0 ppm, and 8.5 ppm to 6.8 ppm at vicinities in the leaf middle, leaf apex, leaf base, and stem over 2 h during respiration, respectively. These decreases of DO are much larger than those measured without the presence of 1-μM Cu2+ (Fig. 2–C). This suggests that O2 was absorbed much faster by the leaf and other plant organs when presented with 1-μM Cu2+ during respiration. On the other hand, DO also decreased from 8.5 ppm to 6.0 ppm in the middle vicinity over a 2-h period of photosynthesis (Fig. 3–D). In the vicinities of the leaf apex, leaf base, stem, and root, DO decreased with time too during photosynthesis in the presence of 1-μM Cu2+. It is well known that O2 production during photosynthesis is greater than O2 consumption during respiration. Therefore, net O2 is produced during photosynthesis from a plant without disturbance (Fig. 2–D). The results in Fig. 3–D show that the presence of 1-μM Cu2+ disturbed the normal photosynthetic activity of the plant, and photosynthesis inhibition by heavy metal ions has been widely reported (Clijsters and Assche, 1985; Ghori et al., 2019). Figs. 3–C and 3–D outline that not only photosynthesis was greatly inhibited, but also some physiological activities were altered to consume a greater amount of O2 for the presence of heavy metal ions. The effects on growth and long-term physiological damage by the heavy metal ions on aquatic plants require further investigation.
The changes in deflection signals over time at the leaf base, stem, and root shown in Figs. 3–A and 3–B were larger than the changes in Figs. 2–A and 2–B. This difference suggests that the presence of 1-μM Cu2+ increased movement of some chemical species across the leaf base, stem, and root surface. In comparison with the large changes of DO between Figs. 2–C and 3–C, deflection changes between Figs. 2–A and 3–A were not considered remarkable. This is because the deflection signal was not proportional to the DO concentration.
Effects of other heavy metal ions—such as 1-μM Ni2+ or Co2+—on physiological activities of aquatic plants were also investigated. Fig. 4 shows the monitoring results of deflection and DO concentrations in the middle vicinity during both respiration and photosynthesis. Without the addition of 1-μM Ni2+ or Co2+, both deflection and DO changed over time during photosynthesis conversely with respiration. However, when 1-μM Ni2+ or Co2+ existed in the culture solution, changes in trends occurred over time with both deflection and DO. Particularly during respiration, DO greatly decreased over time in the presence of 1-μM Ni2+ or Co2+. In addition, DO decrease over time even during photosynthesis. These results are similar to those when in the presence of 1-μM Cu2+. Therefore, the effects of Ni2+ or Co2+ on the physiological activities of aquatic plants were concluded to be similar to those of Cu2+. These results suggest that the presence of heavy metal ions as low as 1 μM greatly alter physiological activities in aquatic plants.

Beam deflection (A and B) and DO concentration (C and D) during respiration (A and C: LED OFF) and photosynthesis (B and D: LED ON) in the middle vicinity of the plant leaf in a 1-μM Ru (II) complex solution containing 1-μM Co2+ or Ni2+. Concentration of DO monitored by the conventional DO sensor in the 1-μM Ru (II) complex solution containing 1-μM Ni2+ is shown in C and D.
Figs. 4–C and 4–D show one example of DO monitored using the conventional DO sensor when in the presence of 1-μM Ni2+. Although DO obtained through the conventional DO sensor decreased with time in both respiration and photosynthesis processes, the decreases were much smaller than those obtained in the middle vicinity by this method. This finding was similar to previous studies (Wu et al., 2017) conducted without the presence of heavy metal ions. This further supports that the present method is much more sensitive than conventional ones using a DO sensor. Regarding to the reproducibility, the changing trends of both deflection and DO over time in Fig. 2, 3, 4 are fully reproducible with different Egeria densa Planch.
Greater changes were observed in both deflection and DO when the concentrations of Cu2+, Ni2+, or Co2+ were higher than 1 μM. Additionally, the presence of nutrients—such as Hyponex medium—affected how much the trends changed during both deflection and DO. Details on the effects of heavy metal ion concentrations and nutrient addition to culture solutions on the stresses incurred by heavy metal ions will be reported at a later stage.
Real time in situ monitoring of DO and material movement-induced beam deflection at various points on an aquatic plant revealed that the largest changes were observed in the middle vicinity of aquatic plant leaves. The second largest changes were noted in the leaf apex. Both DO and deflection changing trends over time during respiration were converse to those observed during photosynthesis without the presence of heavy metal ions. However, these changing trends over time even out and become comparable during both respiration and photosynthesis when in the presence of 1-μM heavy metal ions, such as Cu2+, Co2+, or Ni2+. This suggests that heavy metal ions as low as 1 μM have inflicted stress on aquatic plants. These results indicate that the present method is not only highly sensitive, but also able to distinguish material movement on different parts across the plant surface. Results further highlighted that this method is an effective and useful tool for studying heavy metal stress in plants.
This work was partly supported by Grant-in Aid for Scientific Research (No. 24550109 and No. 20K05572) from the Japan Society for the Promotion of Science (JSPS).