2024 Volume 4 Pages 19-37
2024 Volume 4 Pages 19-37
There has been little research on the pollution of drifted debris in terrestrial ecosystems in coastal areas compared to research focusing on aquatic environment and aquatic organisms. In this study, two species of terrestrial hermit crabs and three species of coastal plants were collected from a polluted site (Nita) and a control site (Furuzamami) in Zamami Island, Okinawa, and analysed to evaluate element contamination in order to evaluate the risk of drifted debris as a source of trace element pollution in terrestrial ecosystems. In both species of terrestrial hermit crabs, Cd was detected at significantly higher concentrations in both the muscle and hepatopancreas of individuals collected from the polluted site. In addition, Pb, often contained in polyvinyl chloride products along with Cd, had higher concentrations in the hepatopancreas of terrestrial hermit crabs in the contaminated site than in the control site. Among the three coastal plants, Pandanus tectorius was the most sensitive to trace element pollution via drifted debris. In general, plants tended to accumulate elements in accordance with their leachability from plastics. This result suggested that trace elements, leaching from drifted debris to soils, accumulated in the plants. These results indicate that leaching rates from drifted plastics have a greater effect on element accumulation in plants than in terrestrial hermit crabs. The degree of influence of the leaching rate of elements from drifting debris differed between the hermit crabs and plants. In addition, the accumulation of elements that were easily adsorbed by plastics was observed in each species. In the environment, drifted debris is potentially hazardous as a medium of contamination to organisms, as it adsorbs various elements during their transport in the ocean.

Plastic pollution in oceans has grown rapidly globally in recent years (Ostle et al., 2019). Because of their high durability, high persistence and low density, plastic debris does not settle in the water but drifts and causes transboundary pollution from long-distance transport (Khordagui and Abuhilal, 1994; Derraik, 2002; Turner, 2016; Acosta-Coley et al., 2019; Matsui et al., 2022). Plastic debris has a direct negative impact on marine organisms owing to accidental ingestion of plastic, injury from entanglement in drifting plastic and suffocation (Boren et al., 2006; Gregory, 2009; Shaikh and Shaikh, 2021; Carreras-Colom et al., 2022; Moon et al., 2022). Plastics also serve as transporters of several chemical contaminants (Teuten et al., 2007). Therefore, exposure to various chemicals from the ingestion of plastics has negative effects.
The drifted debris in Okinawa consists mainly of plastic bottles, fishing nets and buoys (Okinawa Prefecture, 2020; Matsui et al., 2022). Moreover, drifted plastics and Styrofoam become microplastics, fragmented by physicochemical factors, such as ultraviolet rays, heat and waves (Godoy et al., 2019; Cho et al., 2021). Microplastics also act as transporters of elements (Godoy et al., 2019; Ta and Babel, 2020). In particular, elemental adsorption rates tend to increase owing to the increase in the surface area and oxygen content of degraded microplastics (Wang et al., 2020). Trace elements are added as catalysts, biocides, colorants and flame retardants in plastics (Turner, 2016). Furthermore, some elements are adsorbed onto plastics during ocean transport (Holmes et al., 2012; Turner and Holmes, 2015; Jasna et al., 2018). Microplastics settle mainly in sediment (Ding et al., 2021) and can be taken up by detritus-feeding species (Godoy et al., 2019; Ta and Babel, 2020; Cho et al., 2021). Elements adsorbed or contained in microplastics are generally more soluble under low pH conditions, and 55 elements, including highly toxic elements such as As, Cd, Hg and Pb, were found to be eluted at pH less than 1.5 (Hildebrandt et al., 2021). The pH of living organisms is generally low, and most elements adsorbed or contained in microplastics may leach out when taken into the body (Town et al., 2018; Smith and Turner, 2020; Yamaguchi, 2020). Several previous studies have confirmed the accumulation of elements and toxic effects of microplastic uptake in various aquatic species (Jinhui et al., 2019; Patterson et al., 2020; Yuan et al., 2020; Zhu et al., 2020; Martinez-Tavera et al., 2021).
Zamami Island, located 40 km west of Naha City, Okinawa Prefecture, has similarly reported pollution from drifting debris (Okinawa Prefecture, 2019a). In a survey conducted from 2017 to 2018, 0.0006 m3/10 m of drifted debris was observed at Furuzamami in the southeast of Zamami Island, while 1.00 m3/10 m was observed at Nita Beach in the northern part of Zamami Island owing to the northeast monsoon in winter (Okinawa Prefecture, 2019a). Furthermore, the proportion of Styrofoam and plastic products represented 40 out of 50 m3 of the total debris at Nita (Okinawa Prefecture, 2019a). Plastic debris on Nita not only originated from Japan but also included debris from East and Southeast Asian countries, 80% of which were made in China (Okinawa Prefecture, 2019b). Compared to mainland Japan, Zamami Island is less populated and there is no large-scale human activity in the surrounding areas. Therefore, the major pollution pathways in coastal ecosystems are derived from drifted debris.
In coastal ecosystems, bivalves, gastropods and aquatic crustaceans, as well as terrestrial crustaceans such as terrestrial hermit crabs and coastal plants are present. Terrestrial hermit crabs are omnivores that frequently feed on carcasses in coastal ecosystems (Sasaki et al., 2018, 2021) and plants fruits (Okinawa Prefecture, 2019c). Because of their feeding, these species act as scavengers in coastal ecosystems. However, terrestrial hermit crabs are threatened by debris and microplastics present on beaches and shorelines (Lavers et al., 2020; Tanaka et al., 2023). Focusing on feeding habitats, microplastics and polybrominated diphenyl ethers (PBDEs), chemical compounds contained in microplastics, have been detected in terrestrial hermit crabs collected from polluted sites by drifted debris on Zamami Island (Tanaka et al., 2023). However, studies on the accumulation of microplastic-derived elements in terrestrial crustaceans are scarce (Lavers et al., 2020). Similar to terrestrial hermit crabs, coastal plants are components of coastal terrestrial ecosystems. Coastal plants provide shelter for terrestrial organisms in coastal terrestrial ecosystems and food for insects and other herbivores (Tabuchi et al., 2001; Matsushima et al., 2014). Regarding the toxic effects of elements in microplastics on plants, the accumulation of elements leached from microplastics in Brassica campestris was experimentally demonstrated (Jia et al., 2022). Therefore, the coastal plants on Zamami Island may have been affected by trace element pollution from drifted debris.
In this study, a remote island in Okinawa Prefecture was selected as the research site because of the small effects of anthropogenic pollution other than drifted debris. The purpose of this study was to elucidate the element pollution derived from drifted debris in the coastal terrestrial ecosystems using two terrestrial hermit crab species and three coastal plant species. By comparing element concentrations between beaches with high and low pollution from drifted debris on Zamami Island, the effects of plastic pollution and the elemental compositions of the plastics were evaluated. This study is expected to provide knowledge on the effects of element contamination by plastics and the potential hazards of plastics as transporters for elements in coastal terrestrial ecosystems.
Two terrestrial hermit crabs (Coenobita purpureus and Coenobita ruggosus) and three plants (Pandanus tectorius, Scaevola taccada and Heliotropium foertherianum) were collected from Nita and Furuzamami on Zamami Island on October 14th, 2018 (Fig. 1–A). For the two terrestrial hermit crabs, the feeding habits and home range are commonly. The two terrestrial hermit crabs are omnivorous (Tanaka et al., 2023). Furthermore, both species were determined to be useful for comparisons of the distribution of element concentrations between the two sites because of their settlement characteristics at the two sites focused on in this study. As for plants, P. tectorius and S. taccada were commonly to home range in coastal area at each site; therefore, both plants were determined to be useful for comparison of element concentration distributions between the two sites as well as terrestrial hermit crabs. Furthermore, research on the elemental contamination of plants via microplastics is limited to laboratory experiments and is lacking (Jia et al., 2022). Hence, it is important to collect H. foertherianum, in addition to P. tectorius and S. taccada, at the pollution site to conduct a detailed interspecies evaluation. For the collection of this research, terrestrial hermit crabs were collected with the permission of Japan’s Agency for Cultural Affairs, the permission number of 30-4-1034. In addition, H. foertherianum was collected with permission from the Natural Parks Act by Ministry of the Environment (permission number 1810093).

Zamami Island is one of the islands that make up the Kerama Islands in Okinawa (Zamamimura, 2022). The total population of Zamami Island is 597 and it has an area of 6.71 km2. Its main industries are agriculture, livestock breeding, fishing and tourism (Zamamimura, 2022). Ports and settlements are in the southern part of the island, whereas the northern part has few settlements and facilities. Additionally, there are many cliffs in the northern part of the site, and no inflow of domestic wastewater. Therefore, the northern coast has less anthropogenic influence than the southern coast. Nita is a sandy beach on the northern part of Zamami Island, and coastal forests are spreading on the beach. Furuzamami, located in the southeastern part of the island, is a beach destination for tourists. Like Nita, Furuzamami has a coastal forest in the beach, forming a coastal ecosystem like Nita. The land use map is shown in Fig. 1–B, with reference to JAXA (JAXA, 2023). The land use conditions in the vicinity of Nita and Furuzamami are similar and are dominated by bare land and natural forests, such as Evergreen Broad-leaf Forest (EBF). However, the monitoring results at the two sites show that the amount of debris at Nita is 1.00 m3/10 m, which is about 167 times that at Furuzamami with 0.006 m3/10 m (Okinawa Prefecture, 2019b). Plastic accounted for 80% of the litter at Nita and 35% at Furuzamami, metals accounted for 5% at both sites and driftwood accounted for 15% at Nita and 60% at Furuzamami (Okinawa Prefecture, 2019b). The surface geology of the two sites is identical (Okinawa Prefecture, 2019c, 2022). A comparison of trace element concentrations in organisms living at these two sites with different amounts of debris allows an assessment of the impact of element pollution from drifted debris, which is especially abundant in the drifted debris to the Nita coast, on terrestrial ecosystems.
For coastal plants, leaves of P. tectorius and S. taccada were collected from both Nita and Furuzamami in triplicate, and leaves of H. foertherianum were collected from Nita only, in triplicate. Of the terrestrial hermit crabs, five C. purpureus and three C. rugosus individuals were collected from Nita, and five were collected from Furuzamami. Each species was placed in polyethylene bags and transported to the laboratory in a cooler box with coolant. All samples were immediately washed with ion-exchange water in the laboratory, hermit crabs were stored in a freezer (−20°C) and the plants were stored at room temperature until analysis. All the samples were washed again with ion-exchanged water and ultrapure water before analysis. The cleaned crustacean samples were subjected to biometric weight and length measurements using calipers and an electronic balance. The weight of terrestrial hermit crabs was measured after removal from the shoulder shell. The length of terrestrial hermit crabs was measured between the top of the head and bottom of the posterior carapace and abdomen. The weight and length of the two terrestrial hermit crabs collected from Nita and Furuzamami are listed in Table S1. All crustaceans were separated into muscles and hepatopancreas by using a scalpel. Hepatopancreas is the tissue in which elements taken up by the organism are first metabolised, while the muscle is the tissue in which elements are distributed after metabolic action in the hepatopancreas. To evaluate chronic and acute effects simultaneously, analysis of the hepatopancreas and muscle was necessary. Crustacean samples were divided into petri dishes for each individual, and the tissue was weighed using an electronic balance and oven-dried (90°C, 24 h). After determining the dry weight, crustacean samples were powdered using a ceramic mortar and pestle. The plant leaf samples were divided into three parts to make the total mass as equal as possible and dried at 50°C for at least 24 h. The dried plant samples were then shredded into uniform pieces using dissecting scissors and ground into a powder.
Approximately, 0.100 g of the powdered sample was weighed into a vial tube, and 2.0 ml of 61% HNO3 was added to the dried sample and digestion was carried out using a microwave oven (200 W, 20 min). Digestion samples were filtered through ADVANTEC 5C filter paper, transferred to polypropylene test tubes and diluted approximately 250 times with Milli-Q water. The diluted solution was weighed to determine the dilution factor and was used for analysis. Concentrations of 34 elements (7Li, 23Na, 24Mg, 27Al, 39K, 43Ca, 51V, 52Cr, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 66Zn, 71Ga, 75As, 82Se, 85Rb, 88Sr, 89Y, 95Mo, Cd, 115In, 118Sn, 121Sb, 133Cs, 137Ba, 139La, 140Ce, 155Gd, 157Gd, 195Pt, 205Tl, Pb and 209Bi) were analysed using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500cx) with 103Rh as the internal standard. The Cd concentration in the environment was calculated based on the sum of the count values analysed by ICP-MS to evaluate multiple stable isotopes (106Cd, 108Cd and 111Cd). Similar to Cd, Pb concentrations were calculated based on the sum of the count values of multiple stable isotopes (206Pb, 207Pb and 208Pb). In terms of Gd, 155Gd and 157Gd have a greater ability of the two isotopes to absorb thermal neutrons than other Gd isotopes (Sharma et al., 2007; Narmani et al., 2018). As a result, 155Gd and 157Gd have a high risk of anthropogenic sources because of their use in various industries, such as gadolinia (Gd2O3) used for neutron capture therapy and nuclear reactor operation (Yilmaz et al., 2006; Narmani et al., 2018). Therefore, 155Gd and 157Gd were selected for analysis, and their respective concentration values were calculated without adding them together. In the following sentences and figures, only the element names are given because the mass number was the same as previously indicated. When the element concentration was below the detection limit, the method detection limit was used as the MDL for convenience. Elements below the detection limit for all individuals were excluded from the statistical analysis. The MDL of all the elements is given in Table 1. Furthermore, the accuracy of the methods was assessed using the standard reference materials NIST 1577b and SRM1577b (National Institute of Standards and Technology) (Horai et al., 2006; Suzuki et al., 2006).
| ID | 7Li | 23Na | 24Mg | 27Al | 39K | 43Ca | 51V | 52Cr | 55Mn | 57Fe | 59Co | 60Ni | 63Cu | 66Zn | 71Ga | 75As | 82Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDL | 0.000387 | 0.0531 | 0.0219 | 0.00287 | 0.0600 | 0.00470 | 0.00117 | 0.00421 | 0.00915 | 0.00220 | 0.00557 | 0.00450 | 0.0393 | 0.122 | 0.000356 | 0.000377 | 0.0000183 |
| ID | 85Rb | 88Sr | 89Y | 95Mo | 111Cd | 115In | 118Sn | 121Sb | 133Cs | 137Ba | 139La | 140Ce | 155Gd | 157Gd | 195Pt | 205Tl | Pb | 209Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDL | 0.000548 | 0.00452 | 0.00192 | 0.00248 | 0.000270 | 0.00205 | 0.00187 | 0.00110 | 0.00100 | 0.00260 | 0.00322 | 0.00346 | 0.000555 | 0.000611 | 0.000810 | 0.0826 | 0.00552 | 0.00416 |
All statistical analyses were performed using R software version 3.6.1. (R Core Team, 2019) and R studio for windows. For statistical analysis, a heat map analysis was performed after standardisation. Euclidean distance was calculated for the heat map analysis to create a dendrogram. From the heat map analysis, the pattern evaluation of the distribution of element concentrations in each organism was based on the analysis of the element clusters. In addition, interspecific differences in element distribution patterns, between 2 species of terrestrial hermit crabs and 3 plants, and differences in element distribution patterns in each organism between pollution and control sites were also evaluated by heat map analysis. Kruskal–Wallis test was used to test the differences in elemental concentrations among the three plants in Nita. The Dunn test was applied for post hoc tests using the ‘dunn test’ package after the Kruskal–Wallis test. In order to compare of element concentrations between two groups, Mann–Whitney U test was used for plants in Furuzamami and terrestrial hermit crabs in Furuzamami and Nita by package ‘exactRankTests’. The significance level for all statistical hypothesis testing was set as p<0.05.
Trace element concentrations in muscle and hepatopancreas of C. purpureus and C. rugosus at Nita and Furuzamami are shown in Table 2. To evaluate element contamination from drifted plastics, 12 element concentrations (Al, Cr, Mn, Ni, Cu, Zn, As, Cd, Sn, Sb, Ba and Pb), reported to be present in drifted plastics were compared between the two sites based on a previous study that investigated element concentrations in drifted debris in coastal areas of remote islands in Okinawa Prefecture as well as Zamami Island (Yamaguchi, 2015).
| Place name | Species | n | Tissues | 7Li | 23Na | 24Mg | 27Al | 39K | 43Ca | 51V | 52Cr | 55Mn | 57Fe | 59Co | 60Ni | 63Cu | 66Zn | 71Ga | 75As | 82Se | 85Rb | 88Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nita | Coenobita purpureus | 5 | Muscle | Range | <0.000387–0.399 | 8,040–11,600 | 2,150–2,760 | <0.00287–2.91 | 6,840–7,920 | 1,970–3,920 | 0.163–0.233 | 0.800–1.07 | 0.816–1.50 | 89.5–180 | 0.162–0.437 | 0.442–1.26 | 84.7–141 | 213–452 | <0.000356 | 2.15–62.3 | 3.24–4.97 | 3.33–4.93 | 39.1–80.7 |
| Median | 0.163 | 11,100 | 2,580 | <0.00287 | 7,230 | 2,950 | 0.210 | 0.845 | 1.17 | 138 | 0.381 | 0.819 | 113 | 243 | <0.000356 | 19.3 | 4.23 | 4.37 | 54.1 | ||||
| Average±SD | 0.224±0.103 | 10,648±1,324 | 2,546±223 | 2.24 | 7,386±410 | 2,976±680 | 0.200±0.029 | 0.887±0.097 | 1.18±0.29 | 139±30 | 0.350±0.101 | 0.839±0.264 | 113±18 | 288±88 | <0.000356 | 30.2±25.6 | 4.15±0.55 | 4.22±0.58 | 55.4±15.3 | ||||
| Hepatopancreas | Range | <0.000387–0.340 | 3,470–17,500 | 1,100–6,130 | 1.06–5.97 | 1,230–5,710 | 3,100–21,500 | <0.00117–0.256 | <0.00421–1.50 | 13.1–119 | 1,641,230 | 2.21–3.91 | 0.762–6.28 | 496–1,550 | 1,290–2,730 | <0.000356–0.215 | 1.26–34.6 | <0.0000183–4.56 | 1.03–4.49 | 53.5–450 | |||
| Median | 0.153 | 13,500 | 4,250 | 2.36 | 4,150 | 8,650 | 0.143 | 0.775 | 56.7 | 656 | 3.00 | 2.73 | 930 | 1,780 | <0.000356 | 17.0 | 2.82 | 3.04 | 255 | ||||
| Average±SD | 0.213±0.084 | 12,094±4,703 | 4,092±1,749 | 2.82±1.66 | 3,916±1,627 | 11,846±7,926 | 0.182±0.055 | 0.906±0.355 | 65.2±35.8 | 731±421 | 3.13±0.58 | 3.66±2.15 | 1,033±360 | 1,892±477 | 0.212±0.004 | 18.1±13.4 | 3.05±0.94 | 2.67±1.23 | 259±148 | ||||
| Coenobita rugosus | 3 | Muscle | Range | 0.270–0.366 | 7,490–13,000 | 4,740–5,350 | <0.00287–17.4 | 3,850–5,770 | 11,900–24,600 | <0.00117–0.156 | 1.30–2.30 | 1.91–3.56 | 726–1,530 | 0.616–1.06 | 3.58–8.46 | 60.0–100 | 224–292 | 0.0318–0.0528 | 2.86–12.3 | <0.0000183 | 0.432–4.22 | 291–587 | |
| Median | 0.359 | 9,880 | 4,890 | <0.00287 | 4,620 | 14,400 | 0.156 | 1.76 | 3.27 | 903 | 0.966 | 4.27 | 82.0 | 241 | 0.0394 | 4.11 | <0.0000183 | 1.10 | 397 | ||||
| Average±SD | 0.332±0.044 | 10,123±2,256 | 4,993±260 | 17.4 | 4,747±789 | 16,967±5,493 | 0.1144±0.042 | 1.79±0.41 | 2.91±0.72 | 1,053±345 | 0.881±0.191 | 5.44±2.16 | 80.7±16.4 | 252±29 | 0.0413±0.0087 | 6.42±4.19 | <0.0000183 | 1.92±1.65 | 425±122 | ||||
| Hepatopancreas | Range | 0.0610–0.211 | 6,310–8,830 | 2,920–3,770 | <0.00287–7.18 | 2,370–2,700 | 10,800–13,700 | 0.0291–0.129 | 1.05–1.69 | 23.7–46.6 | 721–946 | 1.16–2.45 | 3.04–4.69 | 432–693 | 774–1,450 | 0.0482–0.0925 | 1.64–5.89 | <0.0000183 | <0.000548–1.74 | 221–419 | |||
| Median | 0.0614 | 6,430 | 3,280 | <0.00287 | 2,430 | 11,100 | 0.0404 | 1.22 | 46.6 | 755 | 1.41 | 3.56 | 559 | 1,130 | 0.0841 | 2.21 | <0.0000183 | 0.476 | 266 | ||||
| Average±SD | 0.111±0.071 | 7,190±1,161 | 3,323±348 | 7.18 | 2,500±144 | 11,867±1,302 | 0.0662±0.0447 | 1.32±0.27 | 48.4±21.0 | 807±99 | 1.67±0.56 | 3.76±0.69 | 561±107 | 1,118±276 | 0.0749±0.0192 | 3.25±1.88 | <0.0000183 | 1.11 | 302±85 | ||||
| Furuzamami | Coenobita purpureus | 5 | Muscle | Range | <0.000387–0.114 | 11,300–15,400 | 2,570–3,500 | <0.00287–3.38 | 5,430–6,440 | 5,320–19,100 | 0.261–0.310 | 1.26–1.56 | 1.01–1.73 | 251–911 | 0.157–0.463 | 1.22–5.00 | 142–200 | 287–364 | <0.000356–0.0349 | 2.13–6.57 | 2.30–3.25 | 3.42–4.58 | 103–295 |
| Median | <0.000387 | 13,300 | 3,230 | 1.66 | 5,870 | 7,210 | 0.281 | 1.45 | 1.26 | 346 | 0.196 | 2.45 | 172 | 307 | <0.000356 | 3.28 | 2.91 | 3.93 | 145 | ||||
| Average±SD | 0.113 | 13,180±1,499 | 3,154±310 | 2.12±0.79 | 5,932±337 | 9,764±4,901 | 0.282±0.017 | 1.40±0.11 | 1.27±0.26 | 459±234 | 0.242±0.113 | 2.65±1.27 | 173±20 | 316±26 | 0.0349 | 4.19±1.77 | 2.89±0.33 | 3.91±0.39 | 172±68 | ||||
| Hepatopancreas | Range | <0.000387 | 4,860–14,600 | 1,570–4,510 | <0.00287–5.02 | 1,900–3,430 | 3,330–15,000 | 0.0807–0.288 | 1.13–4.44 | 9.21–34.5 | 175–779 | 0.506–1.18 | <0.0004–4.13 | 202–958 | 605–2,020 | 0.0351–0.168 | 1.18–2.86 | 1.65–3.93 | 1.58–2.66 | 62.4–346 | |||
| Median | <0.000387 | 7,180 | 1,810 | 1.73 | 2,510 | 5,490 | 0.140 | 1.25 | 10.3 | 258 | 0.769 | 1.70 | 490 | 1,240 | 0.0588 | 2.08 | 2.61 | 2.23 | 84.3 | ||||
| Average±SD | <0.000387 | 8,852±3,455 | 2,438±1,098 | 2.62±1.42 | 2,610±550 | 7,014±4,089 | 0.168±0.072 | 2.26±1.36 | 15.0±9.8 | 351±217 | 0.803±0.217 | 2.37±1.06 | 499±262 | 1,259±463 | 0.0775±0.0465 | 1.98±0.58 | 2.75±0.74 | 2.19±0.37 | 135±107 | ||||
| Coenobita rugosus | 5 | Muscle | Range | 0.190–0.279 | 13,000–16,600 | 4,290–5,730 | <0.00287–10.3 | 7,360–9,200 | 8,130–13,400 | 0.186–0.251 | 1.49–2.07 | 1.66–2.69 | 566–893 | 0.559–0.847 | 2.96–4.34 | 170–201 | 305–333 | 0.0308–0.00739 | 2.76–11.6 | <0.0000183 | 2.04–4.78 | 170–269 | |
| Median | 0.217 | 15,500 | 4,620 | 2.38 | 7,580 | 10,600 | 0.222 | 1.76 | 1.73 | 730 | 0.664 | 3.52 | 172 | 325 | 0.0526 | 4.63 | <0.0000183 | 3.31 | 227 | ||||
| Average±SD | 0.230±0.036 | 15,060±1,299 | 4,914±576 | 4.56±3.36 | 8,056±775 | 10,826±1,731 | 0.221±0.021 | 1.78±0.21 | 1.91±0.39 | 739±105 | 0.669±0.099 | 3.57±0.51 | 181±13 | 322±10 | 0.0516±0.0141 | 5.85±3.18 | <0.0000183 | 3.40±1.04 | 228±33 | ||||
| Hepatopancreas | Range | <0.000387–0.0636 | 2,620–5,440 | 938–1,980 | <0.00287–2.40 | 1,410–2,140 | 2,560–4,840 | <0.00117–0.0299 | 0.441–0.580 | 14.3–38.5 | 181–381 | 0.795–1.63 | 0.760–1.81 | 158–294 | 217–626 | 0.0378–0.0787 | 0.932–2.26 | <0.0000183 | 0.297–1.54 | 42.8–96.9 | |||
| Median | 0.0152 | 3,090 | 1,340 | 1.22 | 1,570 | 3,090 | <0.00117 | 0.498 | 17.8 | 251 | 1.23 | 0.898 | 274 | 553 | 0.0506 | 1.53 | <0.0000183 | 0.488 | 71.1 | ||||
| Average±SD | 0.0335±0.0215 | 3,824±1,191 | 1,356±396 | 1.80±0.48 | 1,734±316 | 3,438±904 | 0.0256 | 0.515±0.050 | 22.8±9.0 | 257±74 | 1.26±0.31 | 1.13±0.39 | 254±49 | 489±143 | 0.0545±0.0154 | 1.59±0.56 | <0.0000183 | 0.730±0.480 | 67.1±19.1 |
| Place name | Species | n | Tissues | 89Y | 95Mo | Cd | 115In | 118Sn | 121Sb | 133Cs | 137Ba | 139La | 140Ce | 155Gd | 157Gd | 195Pt | 205Tl | Pb | 209Bi | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nita | Coenobita purpureus | 5 | Muscle | Range | <0.00192 | <0.00248 | 0.0405–0.0998 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 0.172–1.75 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 |
| Median | <0.00192 | <0.00248 | 0.0596 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 0.589 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | ||||
| Average±SD | <0.00192 | <0.00248 | 0.0673±0.0229 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 0.812±0.533 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | ||||
| Hepatopancreas | Range | <0.00192–0.0706 | <0.00248–0.553 | 2.44–7.82 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 1.04–10.9 | <0.00322–0.400 | <0.00346–0.412 | <0.000555–0.209 | <0.000611 | <0.000810 | <0.0826 | <0.00552–2.88 | <0.00416 | |||
| Median | 0.0599 | 0.368 | 4.10 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 4.01 | 0.304 | 0.375 | 0.163 | <0.000611 | <0.000810 | <0.0826 | 0.993 | <0.00416 | ||||
| Average±SD | 0.107±0.081 | 0.450±0.077 | 4.62±1.99 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 4.38±3.49 | 0.325±0.066 | 0.343±0.085 | 0.166±0.036 | <0.000611 | <0.000810 | <0.0826 | 1.95±0.77 | <0.00416 | ||||
| Coenobita rugosus | 3 | Muscle | Range | 0.0140–0.0361 | <0.00248–0.0504 | 0.213–0.322 | <0.00205–0.00901 | <0.00187 | <0.00110 | 0.00777–0.0356 | 3.68–81.0 | 0.00601–0.0466 | 0.00828–9.058 | 0.0221–0.389 | 0.00453–0.219 | <0.00810–0.0182 | <0.0826 | <0.00552–0.964 | <0.00416–0.137 | |
| Median | 0.0345 | <0.00248 | 0.261 | <0.00205 | <0.00187 | <0.00110 | 0.0317 | 69.3 | 0.0431 | 0.036 | 0.346 | 0.0105 | 0.0111 | <0.0826 | <0.00552 | <0.00416 | ||||
| Average±SD | 0.0282±0.0101 | 0.0504 | 0.265±0.045 | 0.00901 | <0.00187 | <0.00110 | 0.0250±0.0123 | 51.3±34.0 | 0.0319±0.0184 | 0.0341±0.0203 | 0.252±0.164 | 0.0123±0.0072 | 0.0111 | <0.0826 | 0.964 | 0.137 | ||||
| Hepatopancreas | Range | 0.0840–0.0892 | 0.133–0.379 | 3.13–11.9 | <0.00205 | <0.00187 | <0.00110 | <0.00110–0.107 | 2.78–23.7 | 0.0546–0.233 | 0.0817–0.183 | 0.0628–0.294 | 0.0203–0.0284 | <0.000810–0.00455 | <0.0826 | 0.168–0.235 | <0.00416 | |||
| Median | 0.0851 | 0.334 | 5.65 | <0.00205 | <0.00187 | <0.00110 | 0.0204 | 14.7 | 0.160 | 0.123 | 0.211 | 0.0279 | <0.000810 | <0.0826 | 0.225 | <0.00416 | ||||
| Average±SD | 0.0861±0.0022 | 0.282±0.107 | 6.89±3.69 | <0.00205 | <0.00187 | <0.00110 | 0.0637 | 13.7±8.6 | 0.149±0.073 | 0.129±0.042 | 0.189±0.096 | 0.0255±0.0037 | 0.00455 | <0.0826 | 0.209±0.030 | <0.00416 | ||||
| Furuzamami | Coenobita purpureus | 5 | Muscle | Range | <0.00192 | <0.00248 | <0.000270–0.0499 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 1.17–6.49 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 |
| Median | <0.00192 | <0.00248 | <0.000270 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 4.75 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | ||||
| Average±SD | <0.00192 | <0.00248 | 0.0466 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 3.82±2.12 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | ||||
| Hepatopancreas | Range | <0.00192–0.0747 | <0.00248 | 0.184–1.70 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 0.848–5.89 | <0.00322–0.145 | <0.00346–0.136 | <0.000555–0.102 | <0.000611 | <0.000810–0.0037 | <0.0826 | <0.00552 | <0.00416 | |||
| Median | <0.00192 | <0.00248 | 1.30 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 1.43 | <0.00322 | <0.00346 | <0.000555 | <0.000611 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | ||||
| Average±SD | 0.0497 | <0.00248 | 1.10±0.52 | <0.00205 | <0.00187 | <0.00110 | <0.00110 | 2.28±1.85 | 0.0977 | 0.0823 | 0.0664 | <0.000611 | 0.0370 | <0.0826 | <0.00552 | <0.00416 | ||||
| Coenobita rugosus | 5 | Muscle | Range | 0.00606–0.0432 | 0.0309–0.0771 | 0.0734–0.126 | <0.00205–0.0271 | <0.00187 | <0.00110–0.0243 | 0.0198–0.0471 | 2.92–18.1 | 0.00391–0.0298 | 0.0118–0.0320 | 0.0287–0.0893 | <0.000611–0.0254 | <0.000810–0.0194 | <0.0826 | <0.00552–0.0662 | <0.00416–0.0335 | |
| Median | 0.0255 | 0.0745 | 0.117 | 0.0129 | <0.00187 | <0.00110 | 0.0397 | 6.66 | 0.0165 | 0.0187 | 0.0499 | 0.0126 | 0.00935 | <0.0826 | <0.00552 | 0.00657 | ||||
| Average±SD | 0.0243±0.0128 | 0.00638±0.0175 | 0.106±0.019 | 0.0144±0.0087 | <0.00187 | 0.0243 | 0.0369±0.0103 | 8.34±5.14 | 0.0165±0.0088 | 0.0216±0.0076 | 0.0520±0.0222 | 0.0153±0.0062 | 0.0124±0.0051 | <0.0826 | 0.131 | 0.0197±0.0110 | ||||
| Hepatopancreas | Range | 0.0108–0.0451 | 0.122–0.212 | 0.539–1.65 | <0.00205–0.00541 | <0.00187–0.405 | <0.00110 | <0.00110–0.0101 | 0.924–6.98 | 0.0108–0.0506 | 0.0162–0.0733 | 0.0150–0.0617 | 0.00414–0.0144 | <0.000810–0.00588 | <0.0826–0.0253 | 0.0490–0.137 | <0.00416–0.00603 | |||
| Median | 0.0212 | 0.177 | 0.718 | 0.00478 | <0.00187 | <0.00110 | 0.00561 | 1.65 | 0.0361 | 0.0571 | 0.0414 | 0.0104 | 0.00546 | <0.0826 | 0.115 | 0.00304 | ||||
| Average±SD | 0.0252±0.0115 | 0.169±0.033 | 0.880±0.396 | 0.00429±0.00124 | 0.405 | <0.00110 | 0.00833±0.00195 | 2.58±2.22 | 0.0366±0.0144 | 0.0523±0.0191 | 0.0425±0.0162 | 0.00923±0.00367 | 0.00511±0.00087 | 0.0253 | 0.104±0.031 | 0.00411±0.00129 |
In C. purpureus, both Sn and Sb concentrations, contained in the drifted plastics (Yamaguchi, 2015), were below the MDL in both muscle and internal organs of all individuals in both Nita and Furuzamami. The elemental concentrations in the muscle contained in the drifted plastics were high in Furuzamami, except for As and Cd (Fig. 2). Especially in muscle, Cr, Ni and Cu were significantly higher in Furuzamami individuals, only Cd was significantly higher in Nita individuals (p<0.05). While, in the hepatopancreas, the element concentrations except Cr were higher for Nita than for Furuzamami (Fig. 3). In the hepatopancreas, Mn and Cd concentrations were significantly higher in the Nita individuals (p<0.05). Different patterns were observed in the muscle and hepatopancreas of C. rugosus at the two sites (Figs. 2 and 3). Munuera et al. (2021) found little effect of microplastics on Pb accumulation in the muscle of the crustacean Callinectes sapidus but a high effect in the hepatopancreas. The element concentrations derived from drifted plastics, including Pb like a previous study (Munuera et al., 2021), were higher in the hepatopancreas of individuals in the more polluted site, Nita. Therefore, the hepatopancreas was more affected by the accumulation of trace elements via microplastics ingested from the environment than the muscles of C. purpureus, as previously found in other crustaceans.


In C. rugosus, all element concentrations except Cu and Zn (Fig. 4) in muscle and all 12 elements (Fig. 5) in hepatopancreas were higher in Nita individuals. However, Al was detected in more individuals in both tissues of Furuzamami than in those of Nita. Compared to C. purpureus, C. rugosus tended to accumulate elements derived from drifted debris in both tissues at the pollution site of Nita. Significant differences were observed in the muscle, with significantly higher concentrations of Cu and Zn in Furuzamami individuals and Cd in Nita individuals (p<0.05). In the hepatopancreas, Cr, Ni, Cu, Zn, Cd and Pb levels were significantly higher in Nita individuals (p<0.05). In terrestrial hermit crabs, the effect of element accumulation by microplastics was higher in the hepatopancreas than in muscular tissues, a feature shared by C. purpureus and C. rugosus. Comparison of element concentrations between C. purpureus and C. rugosus showed Cd, which was commonly detected in muscle and hepatopancreas, was significantly higher in Nita than Furuzamami (p<0.05). About plastics made in China, which accounts for about 80% of the plastic debris that washes ashore on Zamami Island, plastic products are regarded in previous studies as one of the major sources of anthropogenic Cd emissions in China (Shi et al., 2019). In particular, in polyvinyl chloride (PVC) plastic products, Cd occurs at extremely high concentrations (Turner, 2019). Moreover, Cd is added to the production process of PVC products (Zaiour et al., 2014). In addition, PVC plastic toys made in China were found to have higher Cd contents than non-PVC plastic toys in plastic products made in China (Omolaoye et al., 2010). Crustaceans, including terrestrial hermit crabs, accumulate microplastics in their bodies mainly by accidental ingestion during feeding (Crooks et al., 2019; Crump et al., 2020). The microplastics mistakenly swallowed by terrestrial hermit crabs on Zamami Island. Based on the fact that significant differences between the pollution and control sites were both shown for the two terrestrial hermit crabs, the results suggest that drifted debris caused Cd accumulation in hermit crabs and the possibility of drifted debris functioning as a source of Cd pollution. In addition, PVC products should be evaluated as a load factor by conducting further research on the proportion of drifting debris and microplastics because they are plastic products with a particularly high Cd content.
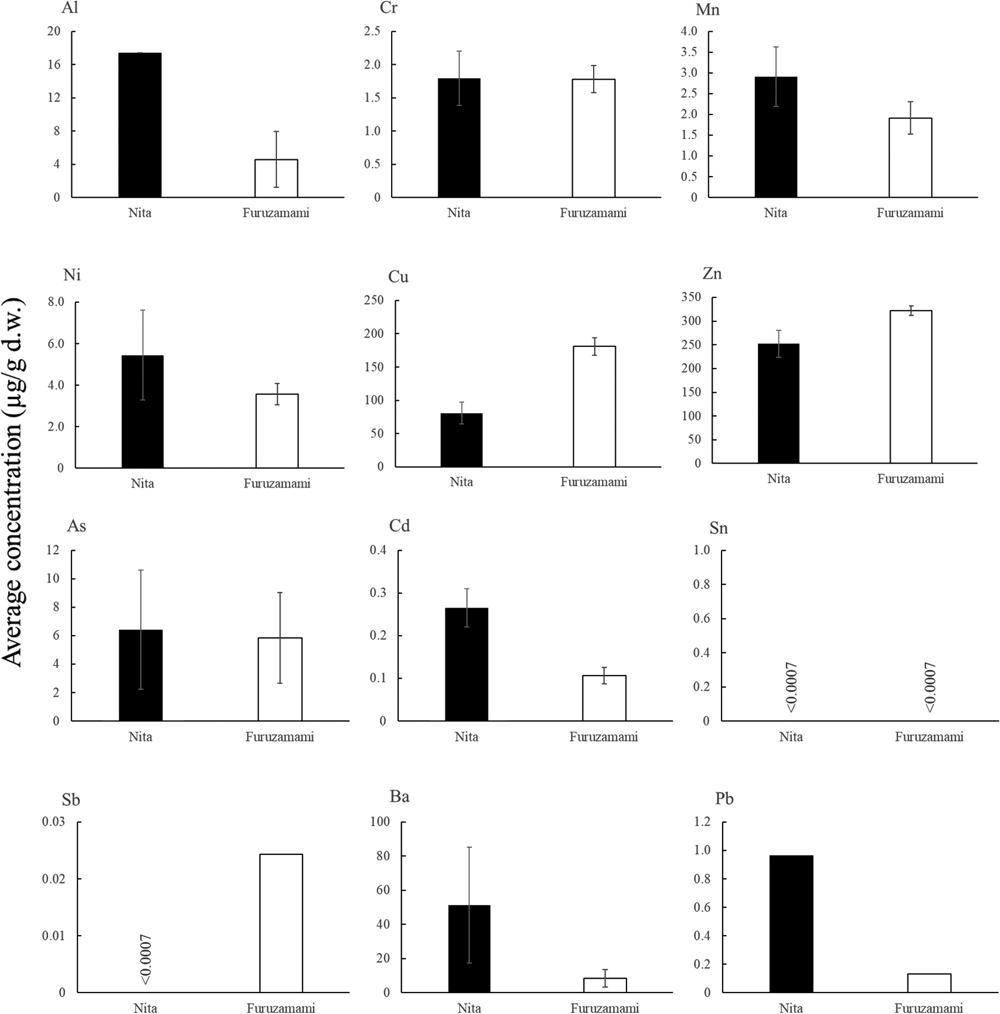

However, in previous study that focused on the elution rate of trace elements from microplastics, Cd was reported to have a low elution rate from plastics (Zhou et al., 2013; Turner, 2018). Moreover, a study on Yonaguni Island, which belongs to Okinawa Prefecture as well as Zamami Island, using the bivalve Atactodea striata to evaluate element contamination from drifted plastics showed no significant difference in Cd concentrations between the contaminated and control sites, indicating Cd contamination may depend on leaching rates (Matsui et al., 2022). The results of Matsui et al. (2022) differed from our results for the terrestrial hermit crabs. Each organism misuses and accumulates microplastics in the body mainly by feeding (Crump et al., 2020; Ding et al., 2020, 2021). However, the main habitats and feeding habits of terrestrial hermit crabs and bivalves are different. Bivalves live in the intertidal to subtidal zone and filter feed on detritus in the water (Liu et al., 2018; Ding et al., 2020, 2021; Oya et al., 2022). Terrestrial hermit crabs live mainly at the base of plants, roam from land to surf and feed directly on fruits, other organisms and carcasses (Sasaki et al., 2018, 2021). It is possible that the main components of microplastics present in detritus and suspended particles in the intertidal to subtidal zones may differ from those present in terrestrial fruits and organisms. In hepatopancreas, Mn, Ni, Cu, Zn and Pb showed significant differences in concentration between the two sites only in one species and were high in Nita. These elements are suggested to be representative elements contaminating terrestrial hermit crabs by drifted plastics on Zamami Island, next to Cd. All of these elements are added to plastics as colour pigments (ECHA, 2019). The common use of colour pigments may have led to contamination of terrestrial hermit crabs with these elements via microplastics and drifted debris.
In contrast, Cr was detected at higher concentrations in individuals at Furuzamami compared to Nita, except in hepatopancreas of C. rugosus. The possibility of Cr contamination in terrestrial hermit crabs via drifting plastic debris was likely to be relatively small. Chromium is used as a colouring pigment, especially as a compound of Pb, PbCrO4 (Turner, 2016). However, the accumulation patterns of Pb and Cr in terrestrial hermit crabs differed between sites. On Yonaguni Island, a remote island in Okinawa Prefecture, and Zamami Island, differences in the accumulation patterns of Pb and Cr in the bivalve Atactodea striata have been demonstrated (Matsui et al., 2022). Unlike Cr, Pb is used as a heat stabiliser for applications other than pigments (Matsui et al., 2022). The difference in the use of Pb and Cr in plastic products is the cause for this difference (Matsui et al., 2022). Furthermore, Cr is more abundant in Styrofoam than in other types of plastics (Klöckner et al., 2020). Unlike Cr, Pb is present in PVC products as a stabiliser against heat and ultraviolet rays (Guardiola et al., 2012; Turner, 2016). Therefore, differences in the accumulation patterns of Pb and Cr may have been influenced by differences in the additive applications and the types of plastic product mainly contained in them. For PVC products with Pb added as a stabiliser against ultraviolet rays and heat, the Cd results may also suggest one of the load factors for the terrestrial ecosystem of Zamami Island. The results for Pb also suggest the need to monitor the terrestrial environment, focusing on PVC as a source of chemical load to organisms, as well as Cd.
The results in the muscles of C. purpureus showed that individuals from Nita and Furuzamami formed different respective clusters (Fig. 6–A). Statistical analysis supported the differences in the accumulation of elements in the muscles of this species between Nita and Furuzamami. Manganese, Cd and As accumulation, known to be present in drifted debris, was characterised in Nita individuals. The Cd concentration was significantly higher in Nita individuals and As also had a higher average concentration in Nita than Furuzamami individuals, although non-significant (Fig. 2). The heat maps showed that As and Cd tended to accumulate in the muscles of C. purpureus at polluted sites, likely due to plastic debris.

Elements Na, Ca, V, Cr, Fe, Ni, Cu, Sr and Ba, characterised in Furuzamami individuals, were significantly more abundant than in Nita individuals (p<0.05). Sodium, Ca, V, Fe and Cu are essential invertebrate elements. In addition, essential elements such as Mg and Zn were also found to accumulate in Furuzamami individuals. In the uptake of essential elements by living organisms, the inhibition of uptake by other elements with similar properties occurs because of competitive effects of binding to the ligands (Di Toro et al., 2001; De Jonge et al., 2014). Competitive effects may also occur during the transfer of essential elements through the channels (De Jonge et al., 2014). For example, highly toxic elements such as Cd and Pb have been shown to be transferred to the body through Ca channels and to compete with Ca ions (Niyogi and Wood, 2004; Rogers and Wood, 2004; Rainbow and Black, 2005). In fact, Cd and Pb are present in drifted plastics in Okinawa Prefecture (Yamaguchi, 2015), and their concentrations were higher at the pollution site than at the control site. According to these results, abnormal tissue distribution of essential elements may be disturbed by plastic debris pollution. In contrast, Furuzamami individuals were characterised by a high accumulation of Ni and Cu, both of which are included in drifted plastics; however, both are also essential elements. The distribution of Ni and Cu in the muscle may have been affected by a similar distribution behaviour to other essential elements.
Similar to C. purpureus, the C. rugosus muscle generally formed different clusters between the two sites for element accumulation (Fig. 7–A). However, more elements were characterised in the polluted site, Nita, than in Furuzamami for C. rugosus. Among the 12 elements reported to be present in drifted plastics (Yamaguchi, 2015), Al, Cr, Mn, Ni, As, Cd, Ba and Pb were characterised by high accumulation in Nita individuals. In contrast to C. purpureus, C. rugosus tended to be characterised by higher concentrations of essential elements in Nita individuals. Among the essential elements, Fe and Co are present in some plastics (Klöckner et al., 2020; Turner et al., 2022). These results suggested that the sensitivity of C. rugosus to plastic-derived elements and their distribution in the muscle tissue may be higher than that of C. purpureus. The essential elements K, V, Cu and Zn showed significantly higher concentrations (p<0.05) and accumulation characteristics in Furuzamami individuals. These elements, except for K, also showed higher accumulation in C. purpureus from Furuzamami than in that from Nita. Copper and Zn are also present in drifted plastics (Yamaguchi, 2015). The results indicated that V, Cu and Zn were easily distributed in the muscle of the terrestrial hermit crabs.

In the hepatopancreas of C. purpureus, the characteristics of trace element accumulation generally differed between the sites, similar to the muscle (Fig. 6–B). Except for Cr, elements known to be included in drifted plastics (Yamaguchi, 2015; Klöckner et al., 2020; Turner et al., 2022) accumulated at high concentrations in Nita individuals. Statistical analysis also supported drifted plastic debris as a possible pathway for element contamination in C. purpureus. Cr accumulation was characterised by the heat map in the Furuzamami individuals. Although, Cr is reported to be present in drifted plastics, other accumulation sources may exist in C. purpureus on Zamami Island. In the bivalve Atactodea striata on Yonaguni Island, Styrofoam drifting debris was a major load source of Cr (Matsui et al., 2022). In the present study, Cd and Pb concentrations, which are likely to contain PVC products rather than other types of plastic products (Omolaoye et al., 2010), were higher at the pollution site than those of control site. Focusing on Cr, Cd, and Pb, which are thought to be derived from drifting debris, the tendency of elemental pollution between bivalves living in the aquatic environment and terrestrial hermit crabs living in the terrestrial environment was different. The composition of the major plastic fragments present in the aquatic and terrestrial environments may be different. However, the composition of plastic fragments between islands in Okinawa Prefecture has not been previous monitored. Furthermore, studies focusing on the differences in plastic debris composition between terrestrial and aquatic environments are lacking. In this study, monitoring including a more detailed elucidation of plastic debris composition between inter-island and terrestrial/aquatic environments is necessary.
In the hepatopancreas of C. rugosus, individuals from Nita and Furuzamami formed different clusters and showed clear differences between sites (Fig. 7–B). In hepatopancreas of C. rugosus, most elements were characterised by high concentrations in Nita individuals. Among the elements characterised in Nita individuals, Na, Mg, K, Ca, Cr, Fe, Ni, Cu, Zn, Sr, Y, Cd, La, Ce, 157Gd and Pb were detected at significantly higher concentrations than in Furuzamami individuals (p<0.05). Chromium, Ni, Cu, Zn, Cd and Pb have been detected in drifted plastics in Okinawa Prefecture (Yamaguchi, 2015). However, other elements are rarely added to plastics. Plastics are known to adsorb metals from marine environments while drifting in the ocean (Ashton et al., 2010; Holmes et al., 2012, 2014; Vedolin et al., 2017; Jasna et al., 2018). Hildebrandt et al. (2021) reported that As, Be, Bi, Cr, Fe, In, Pb, Th, Sn and rare earth elements were adsorbed on microplastics earlier than other elements. Reflecting on the results of previous laboratory studies, drifted plastics adsorb multiple elements in the natural environment and may function as transporters for a wide variety of elements. The possibility of causing accumulation effects in living organisms has also been suggested.
EVALUATION OF TRACE ELEMENT CONTAMINATION IN COASTAL PLANTS FROM DRIFTING DEBRISIn Furuzamami, the median concentrations of Mg, Sr, Cs and Ba were higher in P. tectorius than S. taccada. Other element concentrations, except Tl and Bi, were higher in S. taccada than P. tectorius (Table 3). However, there were no significant differences in element concentrations between P. tectorius and S. taccada, and element accumulation capacity was slightly higher in S. taccada compared to P. tectorius at the control site.
| Place name | Species | n | 7Li | 23Na | 24Mg | 27Al | 39K | 43Ca | 51V | 52Cr | 55Mn | 57Fe | 59Co | 60Ni | 63Cu | 66Zn | 71Ga | 75As | 82Se | 85Rb | 88Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nita | Pandanus tectorius | 3 | Range | 0.325–0.489 | 5,360–6,600 | 4,550–6,190 | 2.29–3.86 | 2,070–7,790 | 5,300–6,170 | 0.348–0.420 | 0.831–1.74 | 3.73–8.39 | 286–322 | 0.740–0.898 | 1.67–2.12 | 3.26–15.3 | 74.0–93.4 | 0.0151–0.202 | 3.11–3.80 | 16.7–19.3 | 2.98–4.58 | 102–181 |
| Median | 0.332 | 5,640 | 5,920 | 2.97 | 3,910 | 5,390 | 0.405 | 1.12 | 6.56 | 293 | 0.820 | 2.09 | 10.5 | 87.6 | 0.0157 | 3.40 | 18.3 | 3.76 | 153 | |||
| Average±SD | 0.382±0.076 | 5,867±531 | 5,553±718 | 3.04±0.64 | 4,590±2,384 | 5,620±391 | 0.391±0.031 | 1.23±0.38 | 6.23±1.92 | 300±16 | 0.819±0.065 | 1.96±0.21 | 9.69±4.95 | 85.0±8.1 | 0.0170±0.0023 | 3.44±0.28 | 18.1±1.1 | 3.77±0.65 | 145±33 | |||
| Scaevola taccada | 3 | Range | 0.536–0.943 | 18,300–27,200 | 5,880–7,080 | 4.99–15.3 | 8,410–13,400 | 6,410–6,690 | 0.452–0.725 | 1.42–1.91 | 9.44–18.9 | 351–397 | 0.546–0.891 | 2.17–2.42 | 6.75–13.5 | 50.5–57.7 | 0.0256–0.323 | 2.18–3.75 | 10.5–19.9 | 8.06–8.80 | 109–138 | |
| Median | 0.658 | 20,900 | 5,920 | 10.5 | 10,800 | 6,650 | 0.552 | 1.45 | 14.5 | 377 | 0.634 | 2.39 | 8.25 | 57.3 | 0.0264 | 2.55 | 12.6 | 8.54 | 137 | |||
| Average±SD | 0.712±0.171 | 22,133±3,737 | 6,293±556 | 10.3±4.2 | 10,870±2,038 | 6,583±124 | 0.576±0.113 | 1.59±0.22 | 14.3±3.9 | 375±19 | 0.690±0.146 | 2.33±0.11 | 9.50±2.89 | 55.2±3.3 | 0.0281±0.0030 | 2.83±0.67 | 14.3±4.0 | 8.47±0.31 | 128±13 | |||
| Heliotropium foertherianum | 3 | Range | 0.721–1.30 | 10,900–21,200 | 4,710–5,350 | 2.60–6.70 | 5,980–11,100 | 7,360–11,000 | 0.338–0.457 | 0.771–1.09 | 9.26–13.4 | 380–647 | 0.512–0.763 | 2.70–4.07 | 11.6–14.7 | 24.7–39.1 | 0.0336–0.0386 | 1.85–2.81 | 9.64–14.2 | 4.09–6.58 | 171–237 | |
| Median | 1.24 | 13,500 | 5,220 | 5.00 | 9,020 | 7,660 | 0.355 | 0.911 | 10.2 | 410 | 0.568 | 2.97 | 11.7 | 25.1 | 0.0378 | 2.21 | 10.8 | 5.57 | 173 | |||
| Average±SD | 1.09±0.26 | 15,200±4,373 | 5,093±276 | 4.77±1.68 | 8,703±2,103 | 8,673±1,650 | 0.383±0.053 | 0.924±0.131 | 11.0±1.8 | 479±119 | 0.614±0.108 | 3.25±0.59 | 12.7±1.4 | 29.6±6.7 | 0.0367±0.0022 | 2.29±0.40 | 11.5±1.9 | 5.41±1.02 | 194±31 | |||
| Furuzamami | Pandanus tectorius | 3 | Range | 0.180–0.316 | 5,510–7,730 | 4,980–6,790 | 2.50–8.07 | 2,200–5,030 | 5,200–8,450 | 0.126–0.140 | 0.903–1.12 | 3.37–5.81 | 234–372 | 0.193–0.464 | 1.40–2.20 | 4.10–6.26 | 47.1–56.3 | 0.0125–0.0130 | 0.789–1.70 | 1.87–3.97 | 1.95–3.26 | 156–225 |
| Median | 0.270 | 6,650 | 5,020 | 3.62 | 2,780 | 8,010 | 0.129 | 0.965 | 4.91 | 354 | 0.331 | 2.19 | 5.62 | 52.2 | 0.0126 | 1.26 | 2.62 | 3.08 | 209 | |||
| Average±SD | 0.255±0.056 | 6,630±906 | 5,597±844 | 4.73±2.41 | 3,337±1,221 | 7,220±1,440 | 0.132±0.006 | 0.996±0.091 | 4.70±1.01 | 320±61 | 0.329±0.111 | 1.93±0.37 | 5.33±0.91 | 51.9±3.8 | 0.0127±0.0002 | 1.25±0.37 | 2.82±0.87 | 2.76±0.58 | 197±29 | |||
| Scaevola taccada | 3 | Range | 0.749–0.910 | 23,700–28,800 | 4,540–5,230 | 7.90–24.1 | 4,310–6,670 | 6,510–9,220 | 0.328–0.431 | 1.69–2.54 | 6.10–10.0 | 321–462 | 0.452–0.656 | 1.74–3.19 | 4.54–7.98 | 46.8–57.3 | 0.0170–0.0279 | 1.68–2.35 | 9.04–10.3 | 3.83–5.73 | 133–236 | |
| Median | 0.874 | 24,200 | 4,710 | 8.74 | 5,320 | 8,110 | 0.338 | 1.77 | 8.73 | 371 | 0.559 | 2.25 | 6.66 | 52.2 | 0.0196 | 2.11 | 9.57 | 5.06 | 164 | |||
| Average±SD | 0.844±0.069 | 25,677±2,295 | 4,827±294 | 13.6±7.4 | 5,433±967 | 7,947±1,112 | 0.366±0.046 | 2.00±0.38 | 8.28±1.62 | 385±58 | 0.556±0.083 | 2.39±0.60 | 6.39±1.42 | 52.1±4.3 | 0.0215±0.0046 | 2.05±0.28 | 9.64±0.52 | 4.87±0.79 | 178±43 |
| Place name | Species | n | 89Y | 95Mo | Cd | 115In | 118Sn | 121Sb | 133Cs | 137Ba | 139La | 140Ce | 155Gd | 157Gd | 195Pt | 205Tl | Pb | 209Bi | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nita | Pandanus tectorius | 3 | Range | 0.00806–0.00977 | <0.00248 | 0.0560–0.150 | <0.00205–0.00528 | <0.00187–0.0652 | <0.00110 | 0.0139–0.0156 | 0.553–0.726 | 0.0106–0.0123 | 0.0171–0.0211 | 0.0109–0.0121 | 0.00231–0.00307 | <0.000810–0.0168 | <0.0826 | <0.00552–0.132 | <0.00416 |
| Median | 0.00873 | <0.00248 | 0.0813 | <0.00205 | 0.0631 | <0.00110 | 0.0140 | 0.726 | 0.0106 | 0.0184 | 0.0114 | 0.00300 | 0.0168 | <0.0826 | 0.0775 | <0.00416 | |||
| Average±SD | 0.00885±0.00070 | <0.00248 | 0.0958±0.0397 | 0.00528 | 0.0642 | <0.00110 | 0.0145±0.0008 | 0.670±0.083 | 0.0112±0.0008 | 0.0189±0.0017 | 0.0115±0.0005 | 0.00279±0.00034 | 0.0168 | <0.0826 | 0.105 | <0.00416 | |||
| Scaevola taccada | 3 | Range | 0.0120–0.0315 | <0.00248 | 0.0378–0.0751 | <0.00205 | <0.00187 | <0.00110–0.0283 | 0.0118–0.0157 | 0.656–1.31 | 0.0199–0.0422 | 0.0342–0.779 | 0.0154–0.0296 | 0.00539–0.0111 | <0.000810 | <0.0826 | <0.00552–0.261 | <0.00416 | |
| Median | 0.0217 | <0.00248 | 0.0537 | <0.00205 | <0.00187 | <0.00110 | 0.0124 | 0.696 | 0.0268 | 0.0483 | 0.0189 | 0.00653 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | |||
| Average±SD | 0.0217±0.0080 | <0.00248 | 0.0555±0.0153 | <0.00205 | <0.00187 | 0.0283 | 0.0133±0.0017 | 0.887±0.299 | 0.0296±0.0093 | 0.0535±0.0182 | 0.0213±0.0060 | 0.00767±0.00247 | <0.000810 | <0.0826 | 0.261 | <0.00416 | |||
| Heliotropium foertherianum | 3 | Range | 0.0122–0.0350 | <0.00248 | 0.0454–0.0997 | <0.00205 | <0.00187–0.0526 | <0.00110 | 0.0161–0.0194 | 1.39–2.14 | 0.00735–0.0253 | 0.0125–0.0374 | 0.0103–0.0232 | 0.00187–0.00693 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | |
| Median | 0.0206 | <0.00248 | 0.0584 | <0.00205 | <0.00187 | <0.00110 | 0.0164 | 1.44 | 0.0171 | 0.0262 | 0.0162 | 0.00441 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | |||
| Average±SD | 0.0226±0.0094 | <0.00248 | 0.0678±0.0231 | <0.00205 | 0.0526 | <0.00110 | 0.0173±0.0015 | 1.66±0.34 | 0.0166±0.0073 | 0.0254±0.0102 | 0.0166±0.0053 | 0.00440±0.00207 | <0.000810 | <0.0826 | <0.00552 | <0.00416 | |||
| Furuzamami | Pandanus tectorius | 3 | Range | 0.00663–0.00912 | 0.156–0.214 | 0.0318–0.0826 | <0.00205 | <0.00187–0.0567 | 0.00807–0.0127 | 0.0105–0.0212 | 0.569–1.03 | 0.00488–0.00742 | 0.0126–0.0166 | 0.00630–0.00866 | <0.000611 | 0.00533–0.00967 | <0.0826 | <0.00552 | <0.00416 |
| Median | 0.00824 | 0.167 | 0.0522 | <0.00205 | <0.00187 | 0.0125 | 0.0188 | 0.968 | 0.00680 | 0.0158 | 0.00858 | <0.000611 | 0.00747 | <0.0826 | <0.00552 | <0.00416 | |||
| Average±SD | 0.00800±0.00103 | 0.179±0.025 | 0.0555±0.0209 | <0.00205 | 0.0526 | 0.0111±0.0021 | 0.0168±0.0046 | 0.856±0.204 | 0.00637±0.00108 | 0.0150±0.0017 | 0.00785±0.00109 | <0.000611 | 0.00749±0.00177 | <0.0826 | <0.00552 | <0.00416 | |||
| Scaevola taccada | 3 | Range | 0.0170–0.0664 | 0.566–0.710 | 0.0859–0.101 | <0.00205–0.0554 | 0.0703–0.0933 | 0.0129–0.0234 | 0.00686–0.0110 | 0.444–0.876 | 0.0302–0.106 | 0.0649–0.225 | 0.0188–0.0672 | 0.00759–0.0286 | 0.00877–0.0119 | <0.0826 | <0.005552–0.176 | <0.00416 | |
| Median | 0.0186 | 0.698 | 0.0921 | <0.00205 | 0.0749 | 0.0150 | 0.00697 | 0.725 | 0.0328 | 0.0662 | 0.0221 | 0.00795 | 0.0118 | <0.0826 | <0.00552 | <0.00416 | |||
| Average±SD | 0.0340±0.0229 | 0.658±0.065 | 0.0930±0.0062 | 0.0291 | 0.0795±0.0099 | 0.0171±0.0045 | 0.00828±0.0193 | 0.682±0.179 | 0.0563±0.0351 | 0.119±0.075 | 0.0360±0.0221 | 0.0147±0.0098 | 0.0108±0.0015 | <0.0826 | 0.176 | <0.00416 |
Comparing the element concentrations in Nita, 11 elements (Co, Zn, As, Se, Sr, Cd, In, Cs, Ba, Pt and Pb) showed higher median or mean concentrations in P. tectorius than S. taccada. Zinc, As, Cd, Ba and Pb were detected in beach litter (Table 3) (Yamaguchi, 2015). A significant difference was also observed between the two species for Zn (p<0.05), which was added to plastics as a colour pigment (ECHA, 2019), flame retardant and heat stabiliser (Murphy, 2001). The leachability of Zn has been shown the highest among the elements in plastic litter in China (Xu et al., 2020). In previous studies (Matsui et al., 2022), elements highly leachable from plastics were noted to be leached from plastics to the bottom sediment by rainfall under weakly acidic conditions, possibly resulting in their transfer to benthic organisms such as bivalves. Similarly, in plants, Zn leached into the sediment by rainfall may have promoted Zn accumulation in the P. tectorius. The elements of concern derived from drifted debris in S. taccada in Nita were Al, Cr, Mn and Ni. These concentrations of these elements were higher than those in P. tectorius (Table 3).
Among the elements of concern derived from drifted debris (Yamaguchi, 2015), Al, Cr, Mn and Ni were more abundant for S. taccada in Nita than in P. tectorius (Table 3). However, the results were similar to those for Furuzamami, with no significant differences observed both in Nita and Furuzamami, and Al, Cr, Mn and Ni accumulation capacities may be consistent with those of P. tectorius and S. taccada. Therefore, leached elements from drifted debris are incorporated into the plant organisms and disrupt their natural accumulation behaviour. Furthermore, the effects of exposure to elements from drifted debris are likely greater for P. tectorius than for S. taccada.
For H. foertherianum collected only from Nita, Li, Ca, Fe, Ni and Ga concentrations were significantly higher than those of P. tectorius (p<0.05); however, no significant differences were found between H. foertherianum and S. taccada for all elements. Nickel is added as a stabiliser against ultraviolet radiation in plastic products (Murphy, 2001). In addition, the leachability of Ni is as high as that of Zn (Xu et al., 2020). Similar to Zn, the high leachability of Ni may have facilitated its diffusion into the sediment and absorption into the organism through the roots.
Comparison of element concentrations in coastal plants in Nita and Furuzamami showed high Cr, Mn, Cu, Zn, As, Cd, Sn and Pb concentrations in P. tectorius in the polluted site (Table 3). These elements are present in drifted plastic debris (Yamaguchi, 2015). In S. taccada, Al, Mn, Ni, Cu, Zn, As and Pb concentrations were higher at Nita than Furuzamami. However, there were no significant differences in the element concentrations between the two sites for either species. Manganese, Cu, Zn, As, and Pb were commonly found at high concentrations in both species in Nita. Manganese has been shown to be highly leachable element from plastics, second only to Zn (Xu et al., 2020; Yamaguchi, 2020). Similarly, Pb and As showed high leachability from microplastics among the 55 elements analysed at pH 4 (Hildebrandt et al., 2021). Therefore, elements at high concentrations in Nita have a common characteristic of high leachability from plastics (Xu et al., 2020; Yamaguchi, 2020; Hildebrandt et al., 2021). The influx of elements into the soil owing to high leachability is suggested to affect the accumulation of elements in plants.
In contrast, Cr, which is moderately leachable (Xu et al., 2020; Yamaguchi, 2020), and Al and Ni, which are shown to be as highly leachable as Zn (Xu et al., 2020; Yamaguchi, 2020), tended to occur at higher concentrations in Furuzamami than in Nita. Copper, shown in high concentrations at Nita in both coastal plants, has lower leachability than Mn, Zn, As and Pb (Xu et al., 2020). Other factors besides high and low leachability were also suggested to influence the pollution load on coastal plants. Manganese, Cu, Zn and Pb, commonly found in high concentrations in Nita individuals, are added to plastics as colour pigments (ECHA, 2019). Copper and Pb are also added as paints to prevent organisms from attaching to the fishing nets (Xu et al., 2020). Moreover, these elements occurred at high concentrations in terrestrial hermit crabs at both sites. The common additives used in plastics may influence the bioaccumulation of elements from plastics in terrestrial organisms, such as coastal plants and terrestrial hermit crabs. Furthermore, Mn, Cu, Zn and Pb, detected at high concentrations in both terrestrial hermit crabs and coastal plants in Nita, are suggested to be particularly polluting elements for terrestrial organisms via drifted debris.
The results from Furuzamami showed that P. tectorius and S. taccada formed different clusters (Fig. 8–A), indicating interspecific differences in the accumulation of the elements. The only four elements characteristic for P. tectorius were Mg, Zn, Cs and Ba, while most of the elements were characteristic for S. taccada. Although the locations of the two plant species were identical in terms of habitat, the families and genera were different; therefore, distinct species differences existed. Therefore, statistical analysis supported the higher trace element accumulation capacity of S. taccada compared to P. tectorius at this unpolluted site.

The heat map for Nita also revealed interspecific differences in elemental accumulation among the three plant species (Fig. 8–B). The Ni, Cu and Ba characteristics of H. foertherianum are thought to be derived from drifted debris. The elements characterised by P. tectorius in Nita were Co, Zn, As, Se, Cd, In, Sn and Pt. Among these elements, Zn, As, Cd and Sn have been reported to be adsorbed and contained in drifted debris. Zinc accumulated at high concentrations in P. tectorius, even in Furuzamami, and may depend on the accumulation ability of P. tectorius. However, As, Cd and Sn were characteristic of S. taccada in Furuzamami. This result supported the characterisation of high accumulation of As, Cd and Sn, reported to be present in drifted debris (Yamaguchi, 2015), in different plant species at pollution and control sites. Therefore, these elements may have accumulated in P. tectorius from drifted debris. In contrast, the elements showing accumulation characteristics in S. taccada were similar at the two sites. The heat maps indicated that S. taccada was less affected by element accumulation from drifted debris than P. tectorius.
Different clusters were formed between Nita and Furuzamami in P. tectorius (Fig. 9–A). The elements derived from drifted plastics with high accumulation characteristics in Nita individuals included Cr, Cu, Zn, As, Cd, Sn and Pb. In contrast, most elements characterised by accumulation in Furuzamami individuals, such as Na, Ca, Fe and Mo were essential for plants. The heat map also supported the existence of elemental contamination via drifted plastics in P. tectorius. Pandanus amaryllifolius, from the same genus as P. tectorius, is used for phytoremediation of heavy metals (Ngadi et al., 2014). The genus Pandanus was considered to have a unique ability to accumulate more elements than other plant species. In addition, at the pollution site of drifted debris in the Zamami Islands, this species-specific ability to accumulate elements may have influenced the accumulation of elements in P. tectorius via drifted debris.

The results for S. taccada also showed different clusters between the two sites (Fig. 9–B), and supported the differences in element accumulation between Nita and Furuzamami. However, only Cu, Zn, As and Ba were the elements derived from drifted debris characteristic of Nita. In addition, elements derived from drifted debris such as Al, Ni, Cr, Cd, Sn and Sb were characteristic of the non-polluted site Furuzamami. The heat maps also supported the possibility that S. taccada is less sensitive to element pollution by drifted plastics than P. tectorius, with no significant difference in the concentrations of each element in S. taccada between the sites.
Another characteristic common to P. tectorius and S. taccada, Mo was detected in all individuals of both plants in Furuzamami but was below the detection limit in all plant individuals in Nita. Molybdenum concentrations in H. foertherianum were below the detection limit in Nita. Molybdenum is an essential element in plants (Kaiser et al., 2005). In plants, Mo is bound to a molybdenum co-factor (Moco), an enzyme that requires Mo and is used by the enzyme (Williams and Frausto da Silva, 2002). Moco is involved in metabolic functions that affect plant growth, such as nitrogen reductive assimilation and sulfur metabolism (Kaiser et al., 2005; Mendel and Kruse, 2012; Bittner, 2014). Moco biosynthesis is inhibited by Cu in vivo (Moorhead et al., 2003; Kuper et al., 2004; Mendel and Bittner, 2006). In Zamami Island, Cu concentrations in P. tectorius and S. taccada were higher at pollution site, Nita than at the control site, Furuzamami. The inhibitory response of Mo metabolism by Cu may have limited the concentration of Mo in the plant. Furthermore, Copper is one of the typical elements contained in drifted plastic debris on remote islands of Okinawa Prefecture (Yamaguchi, 2015). Therefore, drifted debris may have affected the inhibition of Mo accumulation. Further studies are needed to evaluate the effects of litter on the inhibition of essential element metabolism in plants.
The elemental contamination by drifted debris in terrestrial crustaceans and coastal plants in the terrestrial ecosystem of Zamami Island was evaluated. The results of this study indicated that the difference in the amount of drifted debris affected the difference in element distribution in terrestrial hermit crabs and plants in coastal areas.
Focusing on the hepatopancreas of two terrestrial hermit crabs, 12 element concentrations, reported to be present in drifted debris, were higher in both species at the pollution site than at the control site. Unlike a previous study using bivalves (Matsui et al., 2022), even elements with low leaching rates from plastics such as Cd showed significant differences in concentration between polluted and control sites. In terrestrial hermit crabs from Zamami Island, the elements with significantly higher concentrations in the polluted site were Cd and Pb, which were present at higher concentrations in PVC products than in other types of plastics. In terrestrial hermit crabs, the accumulation of elements derived from drifted plastics was influenced more by the plastic species present in the habitat area than by the leaching capacity of elements from plastics. Furthermore, high element concentrations of concern for adsorption to plastics in laboratory experiments (Hildebrandt et al., 2021) have also accumulated in the hepatopancreas of terrestrial hermit crabs in Nita. From the viewpoint of the adsorption of elements in the natural environment, the potential hazard of plastic products as transporters of elements is sufficiently high for concern about accumulation in living organisms.
Of the coastal plants, the element concentration distributions in P. tectorius and S. taccada differed between the pollution and control sites. In particular, As, Cd and Sn, are reported to be present in drifted debris (Yamaguchi, 2015) in different plant species at pollution and control sites. Because the concentrations of these elements in P. tectorius were higher at the pollution site than at the control site, P. tectorius was particularly sensitive to elements derived from drifted plastics. The transfer of elements derived from drifted debris to plants generally depends on the leaching rate of the elements from plastics. Furthermore, the concentration of Mo, an essential element for plants, tended to decrease remarkably in the contaminated beaches. The toxic effects of drifted plastics on plants include not only element pollution, but also the risk of normal growth inhibition.
In this study, elements not reported to be added to plastics tended to have higher concentrations at the pollution site than at the control site. Therefore, this study suggested that plastic products are extremely hazardous transporters that release toxic elements into the aquatic environment and cause unexpected accumulation effects on plants and animals in coastal areas by the adsorption of various elements during the drifting process. The effects of drifted plastics on terrestrial crustaceans and plants may lead to the destruction of coastal terrestrial ecosystems through the development of toxic effects by disrupting the normal accumulation of elements and direct metabolic functions due to microplastics.
We thank Miyabi Totsu for assisting with the analysis of this study. We thank this study funded by The University of Tokyo FSI—Nippon Foundation Research Project on Marine Plastics, Okinawa Prefecture—Project to develop countermeasures to coastal debris in 2018. The Okinawa Prefecture project surveys were conducted with Daisuke Nogami, Nagako Hiki and Yoshiko Ishikawa of Japan NUS Co., Ltd., as trustees.
Table S1, The data of weight and length of two terrestrial hermit crabs, collected from Nita and Furuzamami on October 14th, 2018.
This material is available on the Website at https://doi.org/10.5985/emcr.20230007.