2023 Volume 57 Issue 5 Pages 134-142
2023 Volume 57 Issue 5 Pages 134-142
The spontaneous formation of ribonucleotides on prebiotic Earth is considered an essential step in the origin of life. Phosphorylation of ribose to form ribose 5'-phosphate with boric acid has been reported as a key step in ribonucleotide synthesis. However, the probability of phosphorylation of ribose with mineral phosphate, which is the most abundant form of phosphate on Earth, remains unclear. Carbonate and formate were both widely available compounds on prebiotic Earth and are known to increase the solubility of mineral phosphates. Therefore, the present study investigates the phosphorylation of ribose with apatite in the presence of carbonate or formate. Ribose was phosphorylated preferentially at 5'-hydroxyl when slightly alkaline ribose solution was dried down with hydroxyapatite, urea, boric acid, and formate or carbonate at 80°C for 24 h. Conversely, the yield was limited to less than 10% in the absence of formate and carbonate at the same pH. Dissolution of apatite was substantially increased in the presence of carbonate and formate, allowing the phosphorylation of ribose. These results suggest that ribose 5'-phosphate may have been spontaneously formed in boron-rich evaporative environments on prebiotic earth, expanding the availability of ribonucleotides on prebiotic Earth in addition to the conventional process through ribonucleosides.
Ribonucleic acids (RNAs) are considered the potential molecules that carried gene information and catalyzed biological reactions in ancient life (Benner et al., 1989; Joyce, 1989; Orgel, 2004). Thus, the spontaneous formation of ribonucleotides and their polymerization to RNA on early Earth have been regarded as essential steps in the origin of life. In all the extant lifeforms, ribonucleotide is formed by the phosphorylation of glucose, followed by its conversion to ribose 5'-phosphate and the addition of a nucleobase. In abiotic syntheses, two approaches for ribonucleotide synthesis have been investigated: (1) ribonucleoside synthesis from ribose and nucleobases or small reactive molecules followed by its phosphorylation (Becker et al., 2016; Gull et al., 2015; Powner et al., 2009; Schoffstall, 1976) and (2) ribose phosphate synthesis followed by nucleobase addition or formation (Hirakawa et al., 2022; Sanchez and Orgel, 1970; Suárez-Marina et al., 2019). Ribose can be obtained by the formose reaction from formaldehyde which can be formed from CO2 and H2O by photochemical reaction (Breslow, 1959; Cleaves, 2008; Furukawa et al., 2019; Pinto et al., 1980; Ricardo et al., 2004). In addition, meteorites may have been another source of ribose on prebiotic Earth (Furukawa et al., 2019).
The former ribonucleotide synthesis approach has been extensively investigated, including the direct and indirect syntheses of ribonucleosides and their phosphorylation with orthophosphate (Becker et al., 2016; Furukawa et al., 2015; Kim et al., 2016; Lohrmann and Orgel, 1971; Powner et al., 2009; Xu et al., 2021). Conversely, the latter approach was investigated in the 1960s and 1970s and researchers discovered that the first step was challenging because a part of the ribose was degraded while the remainder was phosphorylated at its 1'-hydroxyl group (Halmann et al., 1969; Sanchez and Orgel, 1970). More recently, phosphorylation of sugars at different from 1'-hydroxyl have been reported (Hirakawa et al., 2022; Hu et al., 2019; Krishnamurthy et al., 2000; Nam et al., 2017). In particular, a high-yield synthesis of ribose 5'-phosphate was achieved by the dry-down of concentrated orthophosphate solution with boric acid and urea (Hirakawa et al., 2022). Boric acid enables the selective phosphorylation at the 5'-hydroxyl group and avoids the consumption of ribose by binding the 2'- and 3'-hydroxyl groups. Therefore, the latter approach currently has the potential to shed light on the prebiotic ribonucleotide formation process.
Apatite is the most abundant phosphate mineral on Earth and the primary source of dissolved phosphate for the ocean. However, the substantially low solubility of this mineral has been regarded as a major obstacle to yielding phosphorylated compounds in prebiotic Earth (Schwartz, 2006). In the first ribonucleotide synthesis approach, the phosphorylation of ribonucleosides using apatite with evaporation of aqueous solutions containing ammonium oxalate (Schwartz, 1972) and formates with urea (Burcar et al., 2016; Schwartz, 1972) has been reported. Further, the phosphorylation of ribonucleosides has also been performed using apatite with carbonate (Lohrmann and Orgel, 1971). The concentrations of dissolved carbonate in the Hadean oceans would have been higher than those today, given the 100 to 10000 times higher CO2 concentration in the early Archean atmosphere compared with that of today (Krissansen-Totton et al., 2018; Morse and Mackenzie, 1998; Walker, 1985). An increased concentration of dissolved phosphate in carbonate-rich Hadean environments has been proposed and discussed previously (Kakegawa et al., 2002; Toner and Catling, 2020). Carboxylic acids can form complexes with Ca in aqueous solutions and increase the dissolution of phosphate minerals (Goyne et al., 2006). The formation of carboxylic acids by geochemical processes has been suggested, including photochemical reactions and meteorite impacts on prebiotic Earth with a CO2-rich atmosphere (Furukawa et al., 2009; Liu et al., 2021; McCollom and Seewald, 2003; Mohammadi et al., 2020). Meteorites could also have been a source of carboxylic acids on prebiotic Earth (Huang et al., 2005; Yuen and Kvenvolden, 1973). However, it remains unclear whether ribose could be phosphorylated with apatite under such conditions. Therefore, the present study investigated the phosphorylation of ribose with apatite by the dry-down of aqueous solutions containing carbonates or formates.
The starting materials used in the ribose phosphorylation experiments were d-ribose (>99.0%; Wako), monoclinic hydroxyapatite (HAP; Ca10(PO6)(OH)2; Wako), urea (>99.0%; Wako), sodium formate (>98.0%; Wako), ammonium formate (>99.995%; Sigma-Aldrich), sodium bicarbonate (>99.5%; Wako), ammonium bicarbonate (>99.5%; Sigma-Aldrich), and boric acid (>99.5%; Wako). Dissolved orthophosphate was prepared by using the following phosphates: phosphoric acid (>85.0%; Wako), sodium dihydrogen phosphate (>99.0%; Wako), disodium hydrogen phosphate (>99.0%; Wako), and trisodium phosphate (>96%; Sigma-Aldrich). Sulfuric acid (95%; Wako) and sodium hydroxide (>95.0%; Wako) were used for pH control. All deionized water was prepared by MilliQ-Intergal (18.2 MΩ·cm; <3 ppb TOC).
Formic acid (>99.0% LC/MS grade; Sigma-Aldrich) and acetonitrile (99.9% LC/MS grade; Wako) were used for ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS; Shimadzu LCMS-8040). d-ribose 5'-phosphate disodium salt dihydrate (>99.0%; Sigma-Aldrich) was used as a referential standard. Referential standards for ribose 2'-phosphate and 3'-phosphate were prepared according to the method reported by Hirakawa et al. (2022).
Nitric acid (69.0%; Kanto Chemical) was used for inductively coupled plasma mass spectrometry (ICP-MS). Sodium dihydrogen phosphate (>99.0%; Wako) and calcium chloride (>95.0%; Wako) were used as referential standards for P and Ca.
Phosphorylation experimentsThe starting solutions contained 20 mM d-ribose, 40 mM boric acid, and 800 mM urea with either hydroxyapatite (2.5–3 mg; HAP) or orthophosphate (160 mM) and either formate (800 mM; HCOONa or HCOONH4) or carbonate (800 mM; NaHCO3 or NH4HCO3). The pH of the starting solution was adjusted to 8 ± 0.2 by NaOH. The starting solution (20 μL) was vortexed, kept for 24 h at room temperature (~20°C), and then incubated at 80°C for 24 h in a 1.5 mL open microtube. The dissolution of phosphate started 24 h before the incubation. For comparison, these experiments were conducted in the absence of carbonate and formate at pH 8 and 5. The experiments with formate were also conducted with the starting solution at pH 6.5.
Five hundred microliters of water was added to the products obtained from the experiments with apatite. This solution was filtered with a 0.22 μm-PTFE membrane filter, and then dried under vacuum. Fifty microliters of water and 1 μL sulfuric acid were added to the dried samples to adjust the solution pH to <2. This solution was incubated at 90°C for 1 h. In the experiments with orthophosphate, 200 μL water and 4 μL sulfuric acid were added to the products to adjust the solution pH to <2. This solution was incubated at 90°C for 1 h. The incubation in acidic solutions liberates a bound borate from ribose and a bound urea from 1'-hydroxyl of ribose, as shown in a previous study (Hirakawa et al., 2022). Acetonitrile was added to the incubated solutions in a sample/acetonitrile ratio of 4/1 and analyzed with UHPLC-MS/MS.
The identification of ribose phosphate was conducted by UPLC-MS/MS (Shimadzu LCMS-8040) using a hydrophilic interaction chromatography column (HILICpak VT-50 2D, 5 μm 2.0 × 150 mm; Shodex Co.) at 60°C. The sample was eluted with 80% 25 mM ammonium formate and 20% acetonitrile at 0.2 mL/min. The ionization of the samples was conducted in the negative mode of electro spray ionization with a 3.5 V interface voltage. Desolvation, source, and heat block temperatures were 250, 120, and 400°C, respectively. The nebulizer and drying gas flows were 2.5 and 10 L/min, respectively. The CID energy and gas pressure were 12 V and 230 kPa, respectively.
The changes in the solution pH in the phosphorylation experiments were estimated with separate incubation experiments, using starting solutions of 40 times larger volumes (i.e., 800 μL). The concentrations of the starting materials were the same as that in the phosphorylation experiments. The solution was vaporized at approximately 7 h of incubation. Thus, the pH at 24 h was measured using the solution prepared by adding 800 μL water to the product.
Apatite dissolution experimentsThe concentrations of P and Ca in the solutions were evaluated with separate experiments and analyses. The 800 μL aqueous solutions containing hydroxyapatite and either formate (10 M; HCOONa or HCOONH4) or carbonate (1 M; NaHCO3 or NH4HCO3) were incubated at 80°C for 24 h in a sealed polypropylene microtube. The pH of these solutions was 7 (H2O) to 8.4 (NaHCO3 and NH4HCO3) before the incubation and 6.8 (H2O) to 9.1 (NaHCO3) after the experiment. The initial pH of the experiment with HCOONH4 was adjusted to 8 with a NaOH solution.
The incubated samples were filtered with a 0.22 μm-PTFE membrane filter, diluted 100 to 1000 times with 1% nitric acid solution, and analyzed with ICP-MS (Agilent 8800). The RF power and sampling depths were 1550 W and 8.0 mm, respectively. The gas flow of the carrier, He, H2, and O2 were 0.99, 5.0, 6.0, and 0.3 mL/min, respectively.
In the separate dissolution experiments, the concentrations of P and Ca in the aqueous solution with HCOONH4 and HCOONa were 30 and 5 times higher, respectively, than those in H2O (Fig. 1). The concentrations of P in the carbonate solutions were an order of magnitude higher than that in H2O, but the concentrations of Ca were less than that of H2O, particularly for NH4HCO3.

Release of phosphate and calcium from hydroxyapatite. Concentrations of P and Ca were measured in aqueous solutions containing formates or carbonates and hydroxyapatite incubated at 80°C for 24 h in a sealed tube. The pH of these solutions ranged from 7 (H2O) to 8.4 (NaHCO3 and NH4HCO3) before incubation and from 6.8 (H2O) to 9.1 (NaHCO3) after experiment.
The phosphorylation of ribose with apatite marginally occurred (0.08 mol% yield) when moderately acidic ribose solution (pH ~5) was dried down with apatite, urea, and boric acid in the absence of carbonate and formate (Fig. 2 and 3a). Conversely, the phosphorylation was negligible (~0.01 mol% yield) when a slightly alkaline ribose solution (~pH 8) was used under the same conditions. The yield was substantially increased to 0.11–0.32 mol% when HCOONa, HCOONH4, or NH4HCO3 were added to the starting material (Fig. 2 and 3a). However, the effect of NaHCO3 was negligible (Fig. 2e and 3a). The phosphorylation of ribose with apatite also occurred with HCOONa or HCOONH4 (0.4 and 0.2 mol%, respectively) when the experiments started with ~pH 6 solutions (Fig. 3d–e). The yields of ribose 5'-phosphate were substantially higher than those of ribose 2'- and 3'-phosphate in these experiments (Fig. 2 and 3).

Identification of product ribose phosphates by UPLC-MS/MS. Mass chromatogram (m/z: 229) with fragmentation pattern of (a) standard ribose 5'-phosphate (R 5'-P), 3'-phosphate (R 3'-P), and 2'-phosphate (R 2'-P), (b) product in experiment with HCOONH4, (c) product in experiment with HCOONa, (d) product in experiment with NH4HCO3, (e) product in experiment with NaHCO3, and (f) product in experiment without formate and carbonate. Yields show each mean value (n = 3) of ribose 5'-phosphate.
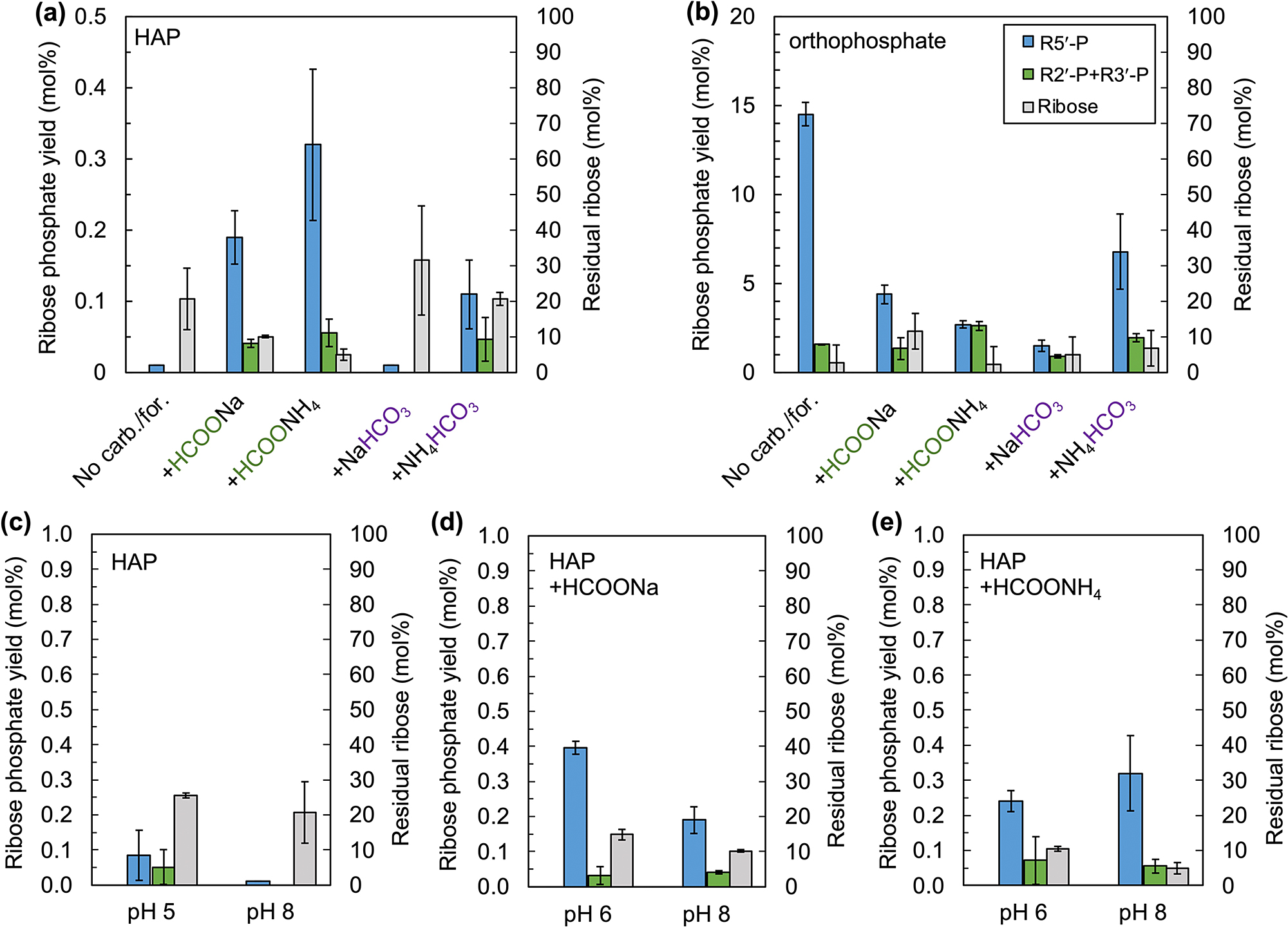
Phosphorylation of ribose with carbonates and formates. (a) Yields of ribose 5'-, 2'-, and 3'-phosphate, and residual amounts of ribose with hydroxyapatite (HAP) from pH 8 and (b) with sodium phosphate from pH 8. (c) Comparison between product ribose phosphates and residual ribose in experiments at pH 5 and 8 in absence of formate and carbonate. (d) Comparison between product ribose phosphates and residual ribose in experiments at pH 6 and 8 with HCOONa. (e) Comparison between product ribose phosphates and residual ribose in experiments at pH 6 and 8 with HCOONH4. Yields show mean values, and error bars represent standard deviation (±1σ, n = 3). R5'-P, R3'-P, and R2'-P represent, ribose 5'-, 3'-, and 2'-phosphate, respectively.
A separate experiment showed that the pH levels of the samples during the incubation were different between the different additives (Fig. 4). In the absence of formate and carbonate, the pH was slightly alkaline at ~8. The same was observed for the experiments with HCOONa or NH4HCO3. The pH of the experiments with NaHCO3 increased to 11.5, while that with HCOONH4 dropped to a moderately acidic pH of ~5.
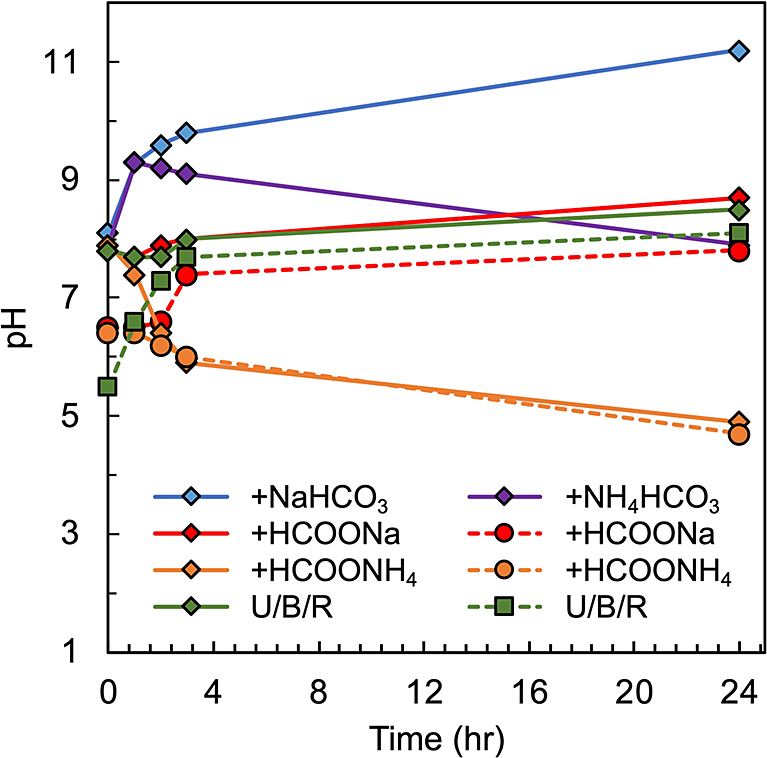
Change in pH of experimental solutions. All the solutions contain U/B/R (urea, boric acid, and ribose). Filled diamonds with solid lines represent the experiments started from pH 8, adjusted by NaOH. Filled circles with dashed lines represent experiments without pH adjustment. Volume of starting solution for pH measurement was 40 times larger than phosphorylation experiments (i.e., 800 μL). Even with larger volume, solutions were completely vaporized at approximately 7 h. Thus, pH at 24 h was measured using solution prepared by adding 800 μL water to product.
In the experiments using starting solutions containing soluble orthophosphate instead of apatite at pH 8, the yield of ribose 5'-phosphate was substantially higher than that with apatite (Fig. 3b). The yield was highest in the experiment where carbonate and formate were not used in the starting material (14.5 ± 0.6 mol%) and the lowest with NaHCO3 (1.5 ± 0.6 mol%) (Fig. 3b). We further assessed the difference in the yields of ribose phosphates between the experiments at different pH with dissolved orthophosphate in the absence of carbonate and formate and found that the yields were pH dependent (Fig. 5). The yields decreased continuously from pH 4 to 12. In this range, the yields were approximately three times higher in the experiments with boric acid. At pH 2, the yield was comparable with that of the pH 4 solution in the absence of boric acid, however that was substantially decreased in the presence of boric acid.

Effect of pH on phosphorylation of ribose. Phosphorylation of ribose was conducted by thermal evaporation of an aqueous solution containing ribose (20 mM), borate (40 or 0 mM), urea (800 mM), and dissolved phosphate (160 mM) at 80°C. pH was adjusted with NaOH. R5'-P, R3'-P, and R2'-P represent ribose 5'-, 3'-, and 2'-phosphate, respectively. Yields show mean values, and error bars represent the standard deviation (±1σ, n = 3).
Ribose reacts first with urea at its 1'-hydroxyl, followed by the complex formation at 2'- and 3'-hydroxyls with boric acid, fixing its form in furanose as discussed in a previous study (Hirakawa et al., 2022). This allowed the remaining hydroxyl (5'-hydroxyl) to regioselectively react with phosphate to form ribose 5'-phosphate as presented in this study (Fig. 6).

Reactions of formation of ribose 5'-phosphate from ribose and apatite with urea and boric acid.
However, the total amounts of the residual ribose and product ribose phosphates are at most approximately 30%, indicating that approximately 70% of the ribose was missing. Ribose is a labile organic compound that can be consumed and converted by many reactions including aldol addition, retro-aldol reaction, epimerization, and disproportionation in solutions (Larralde et al., 1995; Furukawa et al., 2013). In the present experiments, the color of the samples became pale brown after the incubation, suggesting the formation of large molecules by aldol addition. Some of the produced ribose 5'-phosphate may have been consumed by these reactions.
The ICP-MS analysis of the formate or carbonate solutions incubated with HAP indicates that all four formates and carbonates promoted apatite dissolution. Substantially low concentrations of Ca in the dissolution experiments with carbonate indicate that the dissolution of apatite increased with the removal of Ca from the solution as carbonate minerals (Fig. 1). Conversely, in experiments with formates, Ca was present in the solution but formed a complex with formate (Fig. 1). The same has been observed with acetate in a previous study (Bartlett et al., 2018). Formates and carbonates generated more concentrated phosphate solutions than the experiments in the absence of these chemicals at pH 8. Thus, the yields of ribose 5'-phosphate mostly depended on the concentrations of the dissolved phosphate, with the highest yield being obtained for experiments with HCOONH4. It was also observed in the higher amounts of ribose 5'-phosphate in the phosphorylation experiment starting with a moderately acidic solution (pH 5) than with a slightly alkaline solution (pH 8) in the absence of carbonate and formate (Fig. 3c). Phosphate was considerably more dissolved compared to the experiment starting at pH 8, because of the higher solubility of HAP at lower pH (Bell et al., 1978).
The exceptions are the experiments with NaHCO3 in which phosphate was substantially dissolved and the yield of ribose 5'-phosphate was negligible (Fig. 3a and 5). The pH of the experimental solution significantly increased from 8 to 11 (Fig. 4). This affected the yield of ribose 5'-phosphate because the yields of ribose phosphates continuously decreased with pH from 4 to 12 (Fig. 5).
The experiments using orthophosphate as starting material showed lower yields in the presence of formate and carbonate than in their absence (Fig. 3b). In these experiments, much larger amounts of formate and carbonate salts (800 mM) were used than ribose (20 mM) and borate (40 mM). Sodium and ammonium salts of borate have lower solubilities than carbonate and formate salts. Thus, as water vaporized from the reaction vials in the experiments, most of the borate was precipitated as salts, removing it from the system. This likely reduced the yields of ribose 5'-phosphate to be comparable to those of ribose 2'- and 3'-phosphate. This was observed in the reaction between ribose and orthophosphate in the absence of borate which provided comparable yields of ribose 5'-phosphate and ribose 2'- and 3'-phosphates (Fig. 5). Conversely, in the experiments using HAP in the starting material, 5'-phosphate was formed preferentially in the presence of formate and carbonate (Fig. 3a). In these experiments, dissolved phosphate was limiting rather than borate, although a large amount of borate was removed from the system by precipitation. Thus, phosphorylation most likely occurred preferentially at the less sterically hindered 5'-hydroxyl of the ribofuranose-borate complex, yielding regioselectivity.
Implications to prebiotic EarthThe present results indicate that ribose 5'-phosphate may have formed from ribose and phosphate provided by apatite in the boron-rich evaporative environments on prebiotic Earth. The presence of proto-continents on the Hadean Earth was suggested by the oxygen isotope compositions of zircon formed ca. 4.4 billion years ago (Valley et al., 2002; Wilde et al., 2001). Boron-rich shallow sedimentary basins around the proto-continents and proto-arcs have been proposed based on boron isotope compositions in borosilicate minerals found from 3.8 billion-year-old early Archean metasediments (Furukawa and Kakegawa, 2017; Grew et al., 2015; Mishima et al., 2016; Nutman et al., 2015). Boron-rich environments formed, and were possibly widespread, on evaporative basins on proto-continents, in which ribose and urea may have accumulated to potentially high concentrations. The pH of the early Archean ocean is estimated to be moderately acidic to neutral (pH 5.5 to 7) (Halevy and Bachan, 2017; Russell and Hall, 1997). A previous study further suggests that the carbonate-phosphate-rich brine on early Earth was slightly acidic to moderately basic (pH 6.5 to 9) (Toner and Catling, 2020). These pH ranges are compatible with the formation of ribose 5'-phosphate investigated in this study (i.e., pH 5 and 8) (Fig. 4). Borate controlled the phosphorylation site to the 5'-hydroxyl as reported in previous studies (Furukawa et al., 2015; Hirakawa et al., 2022; Kim et al., 2016).
The concentrations of dissolved phosphate in the present dissolution experiments with carbonates and formates were 2 to 20 mM at 24 h incubation in a closed system. Highly concentrated phosphate (up to ~50 mM) can be seen in carbonate-rich lakes on the present Earth, where such concentrations correlate with the concentrations of carbonate (Smith and Stuiver, 1979; Toner and Catling, 2020). Carbonate would have been concentrated in the Hadean boron-rich evaporitic sedimentary basins and lakes because of the high partial pressure of CO2 in the Hadean atmosphere (Toner and Catling, 2020). The concentrations of carboxylic acids in the Hadean open ocean are unclear but should have been lower than that of carbonate. However, it is reasonable to assume that the concentrations of carboxylic acids may have increased in Hadean evaporative basins until carboxylic acids precipitated as salt. Therefore, the Hadean evaporative boron-rich sedimentary basins and lakes around proto-arcs would have been ideal for chemical evolution to facilitate the phosphorylation of ribose to ribose 5'-phosphate. The formation of nucleotides from ribose 5'-phosphate with small reactive molecules or amino acids has also been reported (Sanchez and Orgel, 1970; Suárez-Marina et al., 2019). Further, previous work on the formation of ribonucleosides from ribose and small reactive molecules with a borophosphate mineral provides an insight into the formation of ribonucleotides from ribose 5'-phosphate and small reactive molecules in boron- and phosphate-rich environments when these reactive molecules were supplied (Becker et al., 2019). A recent study further suggests the possible formation of polypeptides in boron-rich evaporative environments on Hadean Earth (Sumie et al., 2023).
The reaction order in the formation pathway of ribonucleotide synthesis investigated in this study contrasts with that of the conventional ribonucleotide synthesis via ribonucleosides. Conversely, the reaction order is more similar to the biological ribonucleotide synthesis in which ribonucleotide is formed via ribose 5'-phosphate. These different pathways do not conflict with each other but expand our understanding of the availability of ribonucleotides on prebiotic Earth.
Ribose 5'-phosphate was formed from ribose and hydroxyapatite in neutral dry-down experiments with boric acid, urea, and formate or carbonate (i.e., HCOONa, HCOONH4, or NH4HCO3). Formate and carbonate contributed to phosphorylation by increasing the dissolution of apatite. Boric acid increased the stability of ribose and controlled the phosphorylation site at the 5'-hydroxyl, bounding 2'- and 3'-hydroxyl of ribose. These results indicate that ribose could be phosphorylated to 5'-phosphate in prebiotic evaporative environments rich in boron. This further suggests that ribonucleotide could be formed through a pathway via ribose 5'-phosphate on prebiotic Earth. This pathway is similar to the biological ribonucleotide synthesis and opposite to that of the conventional pathway in which ribonucleoside is phosphorylated to form ribonucleotide. This pathway expands our understanding of the availability of ribonucleotides on prebiotic Earth, contributing to our knowledge of the potential pathways for the emergence of RNA.
The authors appreciate two anonymous reviewers for their helpful comments on the manuscript. These reviews helped us to improve the manuscript. This work is supported by The Japan Society for the Promotion of Science grant 22H00165 (YF) and 22H00163 (TK). We thank Yoko Nakano of Tohoku University for the ICP-MS analysis.