29th Symposium on Progress in Organic Reactions and Syntheses
Conference information
Host: Division of Organic Chemistry, The Pharmaceutical Society of Japan
Oral Presentations
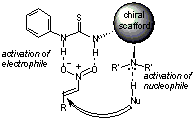
Nucleophilic Addition Catalyzed by Novel Multi-Functional Thiourea Derivatives
Pages 12-13
Details