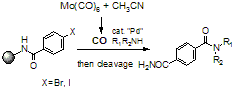29th Symposium on Progress in Organic Reactions and Syntheses
Conference information
Host: Division of Organic Chemistry, The Pharmaceutical Society of Japan
Poster Presentations
Carbonylation Reaction of Aryl Halides on a Polymer Support Using In Situ Generated Carbon Monoxide
Pages 18-19
Details