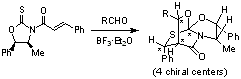29th Symposium on Progress in Organic Reactions and Syntheses
Conference information
Host: Division of Organic Chemistry, The Pharmaceutical Society of Japan
Poster Presentations
Asymmetric Induction of Three Consecutive Chiral Centers by Intramolecular Michael–Aldol Reactions of Thioamide-Enones with Aldehydes
Keywords:
aldol reaction,
asymmetric synthesis,
C–C coupling,
Michael addition,
thiocarbonyl compound
Pages 80-81
Details