29th Symposium on Progress in Organic Reactions and Syntheses
Conference information
Host: Division of Organic Chemistry, The Pharmaceutical Society of Japan
Poster Presentations
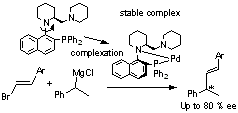
Asymmetric Grignard Cross-Coupling Reaction Using an N–Ar Axially Chiral Mimetic-Type Ligand
Pages 92-93
Details