Abstract
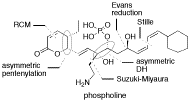
Phospholine is a member of the phoslactomycin family of phosphate ester antibiotics that display potent antitumor activity against a broad range of cancerous cell lines in vitro. Such antitumor activity is attributed to highly potent and selective inhibition of protein phosphatase 2A and 4. Their interesting molecular architectures and the potential as a lead for developing antitumor drugs make these phosphate ester antibiotics attractive targets for synthetic studies. We describe our study toward the enantiocontrolled synthsis of phospholine based on the strategy involving Brown's asymmetric allylation for the introduction of C4 and C5 chiral centers, ring closing metathesis for the construction of the g-lactone moiety, Sharpless dihydroxylation for the assembly of C8 and C9 hydroxy groups, and Suzuki-Miyaura coupling for the attachment of the characteristic C8 aminoethyl substituent.