Abstract
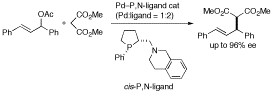
New types of P,N-ligands containing a tetrahydroisoquinoline skeleton as an N-donors were synthesized from P-stereogenic phenyl-P-proline, which we have designed and established an efficient synthetic route. The cis isomer was found to act as an excellent ligand in Pd-catalyzed asymmetric allylic substitution reactions. The reactions of 1,3-diphenyl-2-propenyl acetate with several nucleophiles in the presence of [Pd(π-allyl)Cl]2, cis-P,N-ligand (Pd:ligand = 1:2), and a base afforded the desired products in high yields with high enantioselectivity. It was suggested that these ligands did not serve as a P,N-bidentate ligand but as a P-monodantate ligand in these reactions.