2017 Volume 66 Issue 1 Pages 1-5
2017 Volume 66 Issue 1 Pages 1-5
Transcranial electrical stimulation motor-evoked potential (TES-MEP) has been widely used to monitor major motor pathways in cranial and spinal surgeries. However, the results of TES-MEP might be strongly influenced by anesthetic agents and muscle relaxants. To compensate for this effect, a technique using compound muscle action potentials of the abductor pollicis brevis (APB-CMAP) evoked by median nerve stimulation has recently been reported. In this article, we adopted the transcranial electrical stimulation motor-evoked potential of facial muscles (TES-FMEP) instead of APB-CMAP as a reference waveform for compensation. Intraoperative monitoring in spinal surgeries using TES-MEP, TES-FMEP and APB-CMAP was performed in 64 patients. We compared with and without compensation methods using TES-FMEP and APB-CMAP to evaluate TES-MEP. The cases which demonstrated postoperative motor disturbance, including transient symptoms, were judged to be positive cases. Postoperative transient paraplegia was shown in one intramedullary tumor case among those 64 cases. Compensation by TES-FMEP exhibited the highest specificity (90.5%) and lowest false-positive rate (9.5%) among the three compensation modalities when evaluated at 80% amplitude decrease. TES-FMEP, being derived from motor cortex stimulation, is not influenced by the original spinal lesion or surgical manipulation of the spine. Therefore, compensation using TES-FMEP is suitable for intraoperative monitoring during spinal surgery. The authors advocate TES-FMEP as a reference waveform for the compensation of intraoperative TES-MEP.
Intraoperative neurophysiological monitoring has been widely performed to reduce morbidities such as motor paralysis in spinal surgery6,8,16). Transcranial electrical stimulation motor-evoked potential (TES-MEP) is used to monitor major motor pathways14). Because the administration of muscle relaxants at the induction of general anesthesia strongly affects the waveform of TES-MEP, some kind of compensatory technique to exclude the effect of muscle relaxant is necessary6,8,22). The use of the compound muscle action potential (CMAP) of the abductor pollicis brevis (APB) obtained by stimulation of the median nerve has been reported as a reference waveform of TES-MEP21). However, the origins of TES-MEP are different from those of APB-CMAP: namely, TES-MEP is evoked by stimulation of the motor cortex, whereas APB-CMAP is evoked by stimulus of the peripheral nerves. Moreover, in patients with severe myelopathy causing amyotrophy of the upper extremities, axonal degeneration may make the waveform of APB-CMAP unstable, irrespective of the presence of muscle relaxants15). In skull base surgeries such as those dealing with acoustic neurinoma and petroclival tumors, transcranial electrical stimulation motor-evoked potential of facial muscles (TES-FMEP) has already been used to monitor facial nerve function1,9,10,12). As the entire TES-FMEP stimulus pathway is contained within the cranium, we can hypothesize that TES-FMEP is ideal in that no manipulation during spinal surgery can affect its pathway17). In this article, we evaluate for the first time the validity of TES-FMEP as a reference waveform in spinal surgery.
From September 2009 to January 2015, TES-MEP, APB-CMAP and TES-FMEP monitoring were performed in 64 patients (45 men and 19 women), ranging from 25 to 84 years of age (mean, 59.4 yr) who underwent spinal surgery for 42 cervical, 3 thoracic and 19 lumbar lesions. Patient disorders were varied, as indicated in Table 1. All monitoring was performed using the following system: Viking IV, Viking Select, and Endeavor CR (Nicolet Biomedical Inc., Madison, WI). The muscle relaxant was exclusively administered during anesthetic induction and tracheal intubation, and then total intravenous anesthesia was performed with remifentanil and propofol in all cases. Neuromuscular monitoring was performed by TOF (train-of-four) nerve stimulation. The needle electrodes were placed transcutaneously on the scalp according to the international 10-20 electroencephalogram system20). Transcranial electrical stimulation was accomplished through a pair of needle electrodes (Natus Neurology, Middleton, complete recovery of TOF response applied to the ulnar nerve. TES-MEP was obtained WI) fixed 2 cm anterior to C3 and C4. Two surface electrodes (Natus Neurology, Middleton, WI) were routinely placed at orbicularis oris for recording TES-FMEP. The reference waveforms of TES-MEP, TES-FMEP and APB-CMAP were recorded after by supramaximal stimulation of a TOF stimuli with a 2-ms inter-stimulus interval (ISI). Target muscles of TES-MEP were bilateral abductor pollicis brevis and lower extremity muscles including flexor halluces brevis, anterior tibialis and gastrocnemius. Each TES MEP was simultaneously recorded. Selection of the target muscles of lower extremities was judged a necessity in each case. In TES-FMEP, the ISI was modified to 1-ms because TES-FMEP has a shorter onset latency than TES-MEP. Recording surface electrodes were placed at APB and lower extremity muscles for TES-MEP. APB-CMAP was obtained after stimulation through the electrodes at the wrist: two surface electrodes were placed parallel to the nerve, with the cathode situated distally. Median nerve stimulation was performed by supramaximal stimulation within 50 mA maximum intensity of a single stimulus with 0.2 ms duration. To record APB-CMAP, the same electrodes placed on the APB for TES-MEP were used. APB-CMAP and TES-FMEP were recorded within 5 sec after the recording of TES-MEP. Each waveform was recorded at four points: at the beginning of operation, before and after decompression, and at the end of operation. At the beginning of operation, we confirmed full recovery of train of four ratio. The amplitude of TES-MEP was adjusted by two reference waves: fcMEP (facial-compensated MEP) = TES-MEP divided by TES-FMEP, and acMEP (APB-compensated MEP) = TES-MEP divided by APB-CMAP. The fcMEP and acMEP were calculated immediately after eliciting each waveform during surgery. Amplitude reduction exceeding cut-off value at at least one muscle on each extremity was judged a positive. Manual muscle test (MMT) was employed to evaluate postoperative motor weakness. Detection of at least 1 grade decrease on MMT was judged a positive. Postoperative motor function was evaluated immediately after surgery. The cases which demonstrated postoperative motor disturbance on MMT including transient symptoms were judged to be positive cases. To evaluate correlation between motor weakness and alteration of TES-MEP, we adopted the amplitudes of the beginning and end points of the operation. TES-MEP amplitudes easily fluctuate for various reasons during operation, therefore, we ruled out the values of before and after decompression. All data are expressed as means ± SDs per group and were analyzed by Tukey's honestly significant difference (HSD) test using SPSS 16.0J for Windows (SPSS Inc., Chicago, IL). The false-positive rate (FPR) was calculated using the formula: FPR = 1 − specificity. Statistical significance was assessed using two-way repeated-measures ANOVA for the value of perioperative amplitude and onset latency. Student’s t-test was used to assess the change ratio of amplitude and onset latency after compensation. Statistical significance was determined as a p-value < 0.05. The protocol for the human study was approved by the Institutional Review Board of Hiroshima University.

Distribution of object disorder and region
TES-MEP during spine surgery was recorded from 300 muscles in 64 cases. TES-MEP, TES-FMEP and APB-CMAP were recordable in all cases. No case was complicated with postoperative motor paralysis. Detailed waveform data of TES-FMEP and APB-CMAP are summarized in Table 2. The change ratio of amplitude and onset latency of TES-FMEP and APB-CMAP between the beginning and of surgery had no significant change. The perioperative behavior of amplitude of TES-FMEP and APB-CMAP showed no significant difference (Fig. 1).
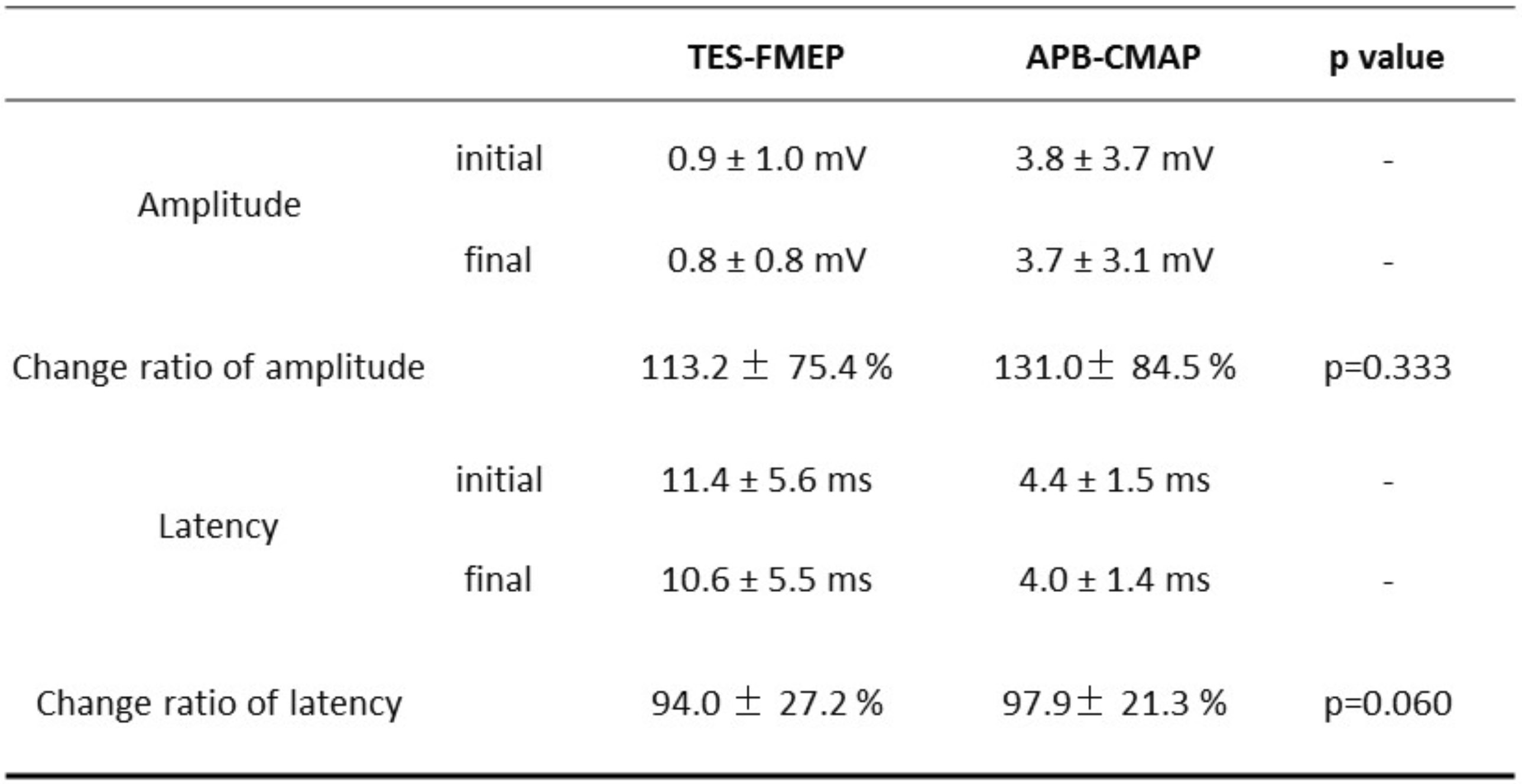
Comparison of reference waveform of amplitude, onset latency and relative value

Perioperative amplitude behavior of APB-CMAP and TES-FMEP.
Each waveforms were recorded at four point: at the beginning of operation, before and after decompression, and at the end of operation. A significant value for Mauchly’s test of sphericity indicates that the assumption of sphericity has been violated. We can report that when using an ANOVA with two-way repeated measures with a Greenhouse–Geisser correction, there are no interaction effects between the longitudinal evaluation of the amplitude of APB- and facial-CMAP.
A, beginning of operation; B, before decompression; C, after decompression; D, end of operation.
a, APB-CMAP; b, TES-FMEP
One intramedullary tumor case demonstrated postoperative transient paraplegia. Amplitudes of lower extremities were decreased during the dissection of the tumor bed from the spinal cord. The operation continued on the basis of fact that reduction in amplitude compensated by fcMEP were >50% but <80%. The neurological symptoms had resolved completely one week following tumor resection. When we set >50% reduction in amplitude as critical, the sensitivities of all methods were 100%, while the specificities were as follows: TES-MEP, fcMEP, and acMEP were 68.3%, 61.9%, and 61.9%, respectively. The false-positive rates were as follows: TES-MEP, fcMEP, and acMEP were 31.7%, 38.1%, and 38.1%, respectively. With cut-off value of >80% reduction in amplitude as critical, the sensitivities of all methods were 100% and the specificities were as follows: TES-MEP, fcMEP, and acMEP were 87.3%, 90.5% and 84.1%, respectively. The false positive rates were as follows: TES-MEP, fcMEP, and acMEP were 12.7%, 9.5% and 15.9%, respectively (Table 3). These results indicate that a > 80% reduction in compensated amplitude should be a critical alarming line in our institute and under this regime, compensation by fcMEP can be regarded as the most reliable method.

False positive rate on amplitude of MEP
A 69-year-old man presenting with intermittent claudication without motor weakness underwent laminectomy for L4-5 canal stenosis. During surgery, TES-MEP recorded in the right gastrocnemius muscle showed an 84% reduction in amplitude. With regard to the compensated data, fcMEP and acMEP were 60% and 75%, respectively. TES-MEPs from bilateral anterior tibialis and left gastrocnemius also demonstrated the same tendency. Because the reduction of the compensated amplitude was < 80%, we judged that the decrease in the amplitude was not critical and continued the operation. The patient did not demonstrate any motor weakness postoperatively (Fig. 2).

Amplitude change of target muscle and reference waveforms.
Amplitudes of the lower extremities became small as the operation progressed. At the same time, the amplitude reduction of the reference waveforms was slight. For example, the muscle MEP of right gastrocnemius decreased to 16%; however, the amplitude compensated by TES-FMEP and APB-CMAP were 40% and 25% of the baseline value, respectively.
Intraoperative neurophysiological monitoring has been widely used to reduce morbidity in spinal surgery6,8). In particular, TES-MEP has become a standard monitoring tool after the introduction of total intravenous anesthesia with propofol and opioid agents4). The problem remains that anesthetic agents such as muscle relaxant may significantly affect the amplitude and latency of MEP6,8,22). Although muscle relaxant is used exclusively at the induction of general anesthesia in our series, it is easy to speculate that a remnant relaxant may reduce the amplitude of MEP at the beginning of the operation. Thus, proper interpretation of the altering waveform requires some kind of compensatory technique. In particular, for those surgeries where continuous administration of a muscle relaxant must be used for some reason, a compensatory technique is required. Such a compensatory technique using APB-CMAP evoked by median nerve stimulation has recently been reported21). Although APB-CMAP is technically easy, the origin of its evoked potential is significantly different from that of TES-MEP. APB-CMAP is derived from the stimulation of the peripheral nerve, whereas TES-MEP is evoked by stimulating the motor cortex. When myelopathy is severe enough to demonstrate amyotrophy of the upper extremities, axonal degeneration may make the waveform of APB-CMAP unstable, irrespective of the presence of muscle relaxant15). Facial nerve motor endplate is resistant to muscle relaxant, however recovers more rapidly from nondepolarizing muscle relaxant2,3). In this study, all evoked potentials were obtained after complete recovery of TOF response, eliminating the effect of muscle relaxants. Moreover, anesthetic fade is mentioned as a factor influencing MEP amplitude. Administration of desflurane/N2O/narcotic or desflurane/propofol/narcotic suppress MEP dependent to duration under anesthesia13). In this study, remifentanil and propofol without volatile agents were adopted for maintenance anesthesia. The suppressive effect of remifentanil on MEP under inducing surgical anesthesia was far less marked18). Although effect of anesthetic fade on TES-FMEP is unclear, we suppose that is negligible under our anesthesia for the above reason. A compensation technique using transcranially evoked CMAP from the sternocleidomastoideus muscle (TC-SCMMEP) as a reference waveform in spinal surgery has been reported23). However, the nucleus of the accessory nerve is widely distributed from the medulla oblongata to the fifth or sixth segment of the cervical cord19). Therefore, original cervical cord pathology or surgical manipulation of the cervical cord may influence the waveform of TC-SCMMEP. TES-FMEP, TES-MEP, and TC-SCMMEP are all derived from motor cortex stimulation. TES-FMEP has already been applied to monitor facial nerve function during surgery for skull base lesions such as acoustic schwannomas and petroclival meningiomas1,10,12). Because the entire TES-FMEP stimulation pathway is enclosed within the cranium, we can estimate that a reference wave obtained by TES-FMEP is not affected by any manipulation during spinal surgery. Since the origin is different, it is insignificant to compare the absolute value of amplitude and onset latency of TES-FMEP and APB-CMAP. We demonstrated that a comparison in the form of relative amplitude was useful in evaluating the intraoperative behavior of TES-MEP. Because there was no significant perioperative behavior in the amplitude of TES-FMEP and APB-CMAP, TES-FMEP can be used as a reference waveform for compensation that shows no inferiority to APB-CMAP. Therefore, we consider that TES-FMEP can be used as a reference waveform for compensation in place of APB-CMAP.
In fact, in this study fcMEP demonstrated the highest value of specificity and the lowest false-positive rate when evaluated at an 80% amplitude reduction, compared with both TES-MEP and acMEP. It has been reported that the threshold for motor palsy in TES-MEP compensated by APB-CMAP was a > 80% reduction in the amplitude21). In the present study, the threshold of fcMEP for motor palsy was assumed to be the same as that of acMEP because the behavior of TES-FMEP showed the same tendency as APB-CMAP.
The question remains as to whether TES-FMEP obtained by our technique is truly derived from the motor cortex. The current spread running outside of the skull may directly stimulate the facial nucleus in the brainstem or facial nerve outside the cranium. To avoid current spread and stimulate the motor cortex effectively, we placed the tip of a needle electrode transcutaneously on the surface of the calvarium11,24). Low-voltage stimulation under 400 mV exclusively excites the motor cortex and avoids motion artifacts of the trunk and extremities. Because the cranial nerves, including the facial nerve, are readily activated under general anesthesia, single-pulse stimulation does not induce a response. Thus, it is important to confirm that single-pulse stimulation at the same intensity as that used in TES-FMEP does not induce a response from the facial nucleus9). Activation of the facial nucleus in the brainstem requires the temporal summation of a series of descending corticobulbar action potentials5,7). In our study, TES-FMEP was recorded with a mean onset latency of 11.4 ms. That is longer than 6.0 ms which is a latency of the direct stimulation of the facial nerve at the CP angle6). If TES-FMEP is recorded with an onset latency under 6 ms, it might be evoked from the brainstem stimulated by current spread. Therefore, TES-FMEP in our study is concluded to be of motor cortical origin.
Because of its short onset latency, recording of TES-FMEP is relatively difficult. When we chose an ISI of 2 ms, the waveform of TES-FMEP could be superscribed by a stimulation artifact. Thus, we modified ISI to 1 ms because such a short stimulatory duration prevents superscription9).
We describe a novel technique of intraoperative compensation of TES-MEP using TES-FMEP in spinal surgery. In this article, fcMEP demonstrated more reliable results than the raw data of TES-MEP and acMEP. We conclude that compensation using TES-FMEP is suitable for intraoperative monitoring during spinal surgery. Although accumulation of clinical data is mandatory, compensation of TES-MEP with TES-FMEP appears to be a good modality of choice in spinal surgery.