2018 Volume 67 Issue 1 Pages 21-29
2018 Volume 67 Issue 1 Pages 21-29
Primary cerebral malignant melanoma accounts for 1% of all melanomas and for 0.7% of all primary tumours of the central nervous system (CNS). We report an incidentally found primary malignant melanoma in the right temporal lobe of a 76-year-old woman with an Ota nevus on her right eyelid and sclera. The lesion was initially characterised by slow growth, followed by tumoural bleeding and wide leptomeningeal dissemination. Magnetic resonance imaging (MRI) during brain checkup demonstrated an 8-mm mass in the right uncus with high intensity on T1-weighted images. The mass grew slowly over the next two years, but asymptomatic tumoural haemorrhage eventually developed. The patient underwent tumour removal via right frontotemporal craniotomy. Extensive subarachnoid dissemination from the right temporal tumour was found; the pathologic diagnosis was malignant melanoma. Despite adjuvant therapy comprising whole-brain radiation and nivolumab, the patient died of severe leptomeningeal dissemination at 4.5 months after the operation (33 months after the initial MRI study). Our literature review found 46 cases of primary CNS malignant melanoma, predominantly in middle-aged to elderly individuals. The most frequent symptom was headache, followed by visual disturbance, nausea/vomiting, and hemiparesis. Only one other case of primary cerebral malignant melanoma had been found incidentally; 11 previously reported patients manifested congenital nevi (Ota nevus, n = 5; other nevi, n = 6). Six patients suffered tumoural haemorrhage, 4 experienced leptomeningeal dissemination, and 5 developed extracranial metastases. The median survival time of the patients was 31 months.
Primary malignant melanoma of the central nervous system (CNS) is a rare tumour. It arises from leptomeningeal melanocytes and accounts for 1% of all melanomas and 0.7% of all primary CNS tumors8,26). Most patients present with increased intracranial pressure, neurological deficits, and seizures33), but asymptomatic cases also rarely occur36).
We report an incidentally found primary malignant melanoma in the right temporal lobe of a woman previously treated for an Ota nevus. The lesion was initially characterised by slow growth, followed by tumoural bleeding and wide leptomeningeal dissemination. We also present a literature review of patients with primary CNS malignant melanoma.
A 76-year-old woman had received YAG laser treatment 10 years earlier for an Ota nevus on her right eyelid and sclera (Figure 1). Without particular symptoms, she subsequently underwent a brain checkup. The examination revealed a single, 8-mm diameter round mass in the right uncus. On magnetic resonance imaging (MRI), the lesion was hyperintense on T1- (Figure 2A) and hypointense on T2-weighted images (T1WI, T2WI). Follow-up MRI performed at a local clinic 11 months after the initial study revealed slight enlargement of the lesion (Figure 2B), suggesting cavernous angioma. Subsequent follow-up showed further growth (Figures 2C, D). The tumour diameter was 20 mm (Figures 3A, B) 24 months after the initial brain checkup. It had very low signal intensity on T2WI scans (Figure 3C) and strongly enhanced with gadolinium (Figure 3D). As these findings indicated an aggressive tumour, the patient was referred to us for further evaluation and treatment. Her admission was postponed owing to the lack of symptoms.
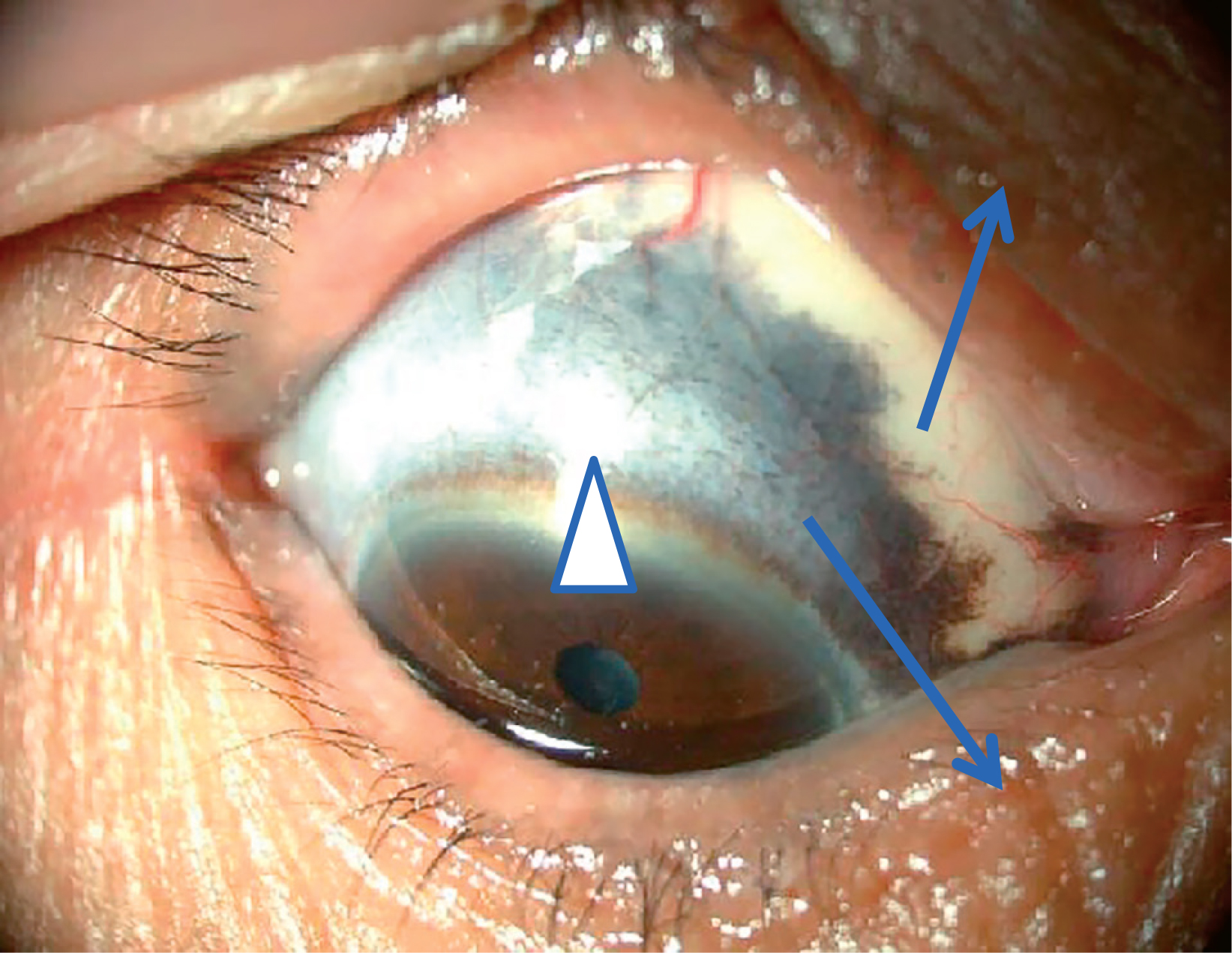
Ota nevus on right eyelid (arrows) and sclera (arrow head)
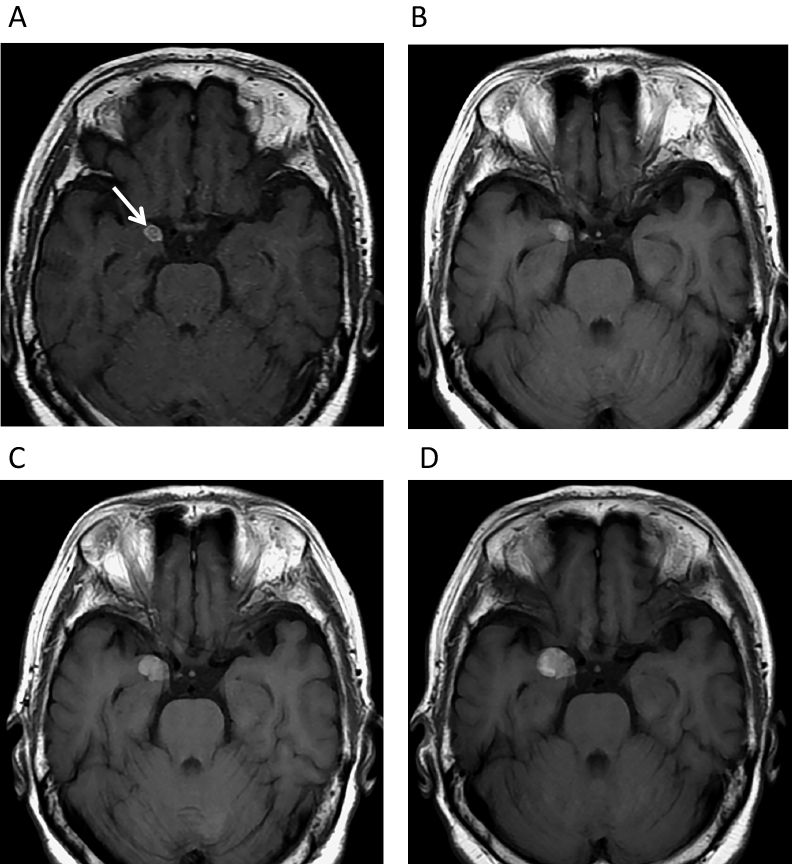
Magnetic resonance imaging (MRI) studies performed before admission to our hospital. (A) September 2014. Axial T1-weighted imaging (T1WI) revealed an 8-mm diameter high-intensity mass (arrow) in the right uncus. Follow-up T1WI demonstrated gradual enlargement of the lesion (B: August 2015, C: February 2016, D: August 2016).
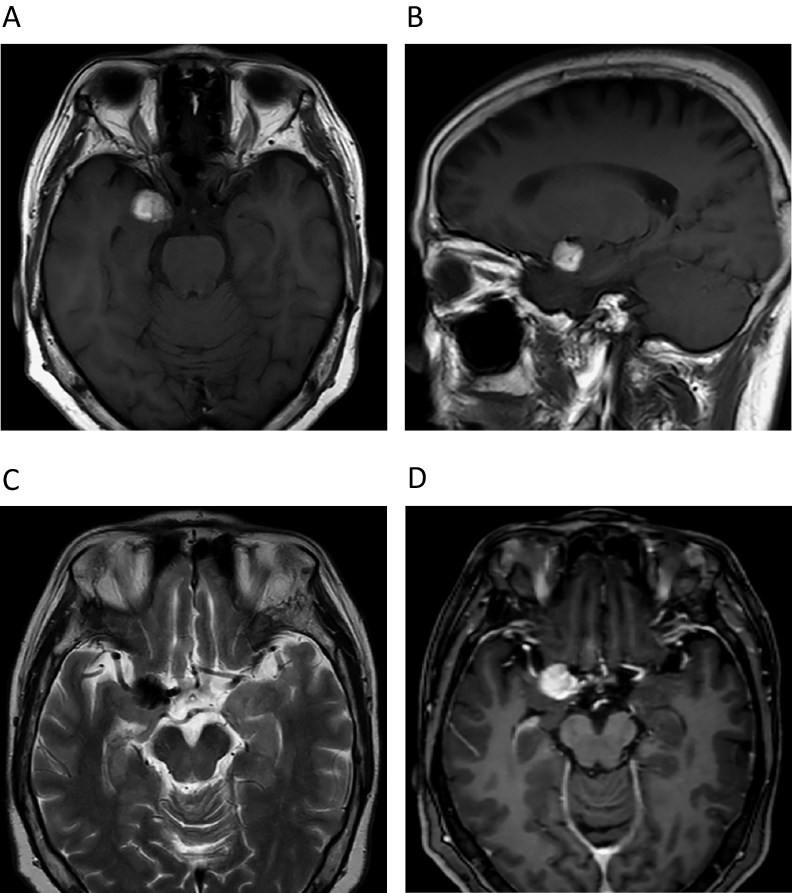
MRI study obtained in September 2016 (24 months after first study). T1WI shows hyperintense mass at right uncus. Mass was 20 mm in maximum diameter (A: axial scan, B: sagittal scan), very low intensity on T2WI scans (C), and strongly enhanced with gadolinium (D).
At the time of admission to our hospital 28 months after the initial MRI scan, the patient was symptom-free. Computed tomography (CT) scan revealed haematoma surrounding the tumour (Figure 4), and the patient underwent right anterior temporal craniotomy. The brain surface was covered by innumerable black spots suggestive of disseminated malignant melanoma (Figure 5A). The temporal lobe tumour exposed by the trans-Sylvian approach was pitch-black with interlacing haemorrhage (Figure 5B). The main mass was removed completely without sequelae.
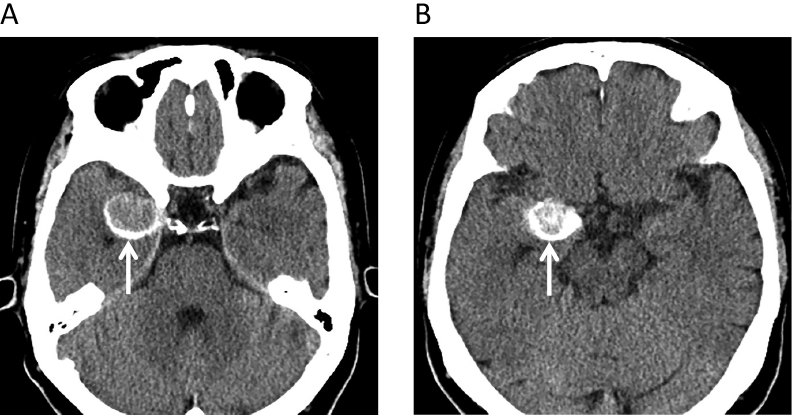
Preoperative computed tomography (CT) images obtained in January 2017. Axial CT shows a round isodense lesion in right uncus surrounded by thin haematoma (A, B) (arrows).
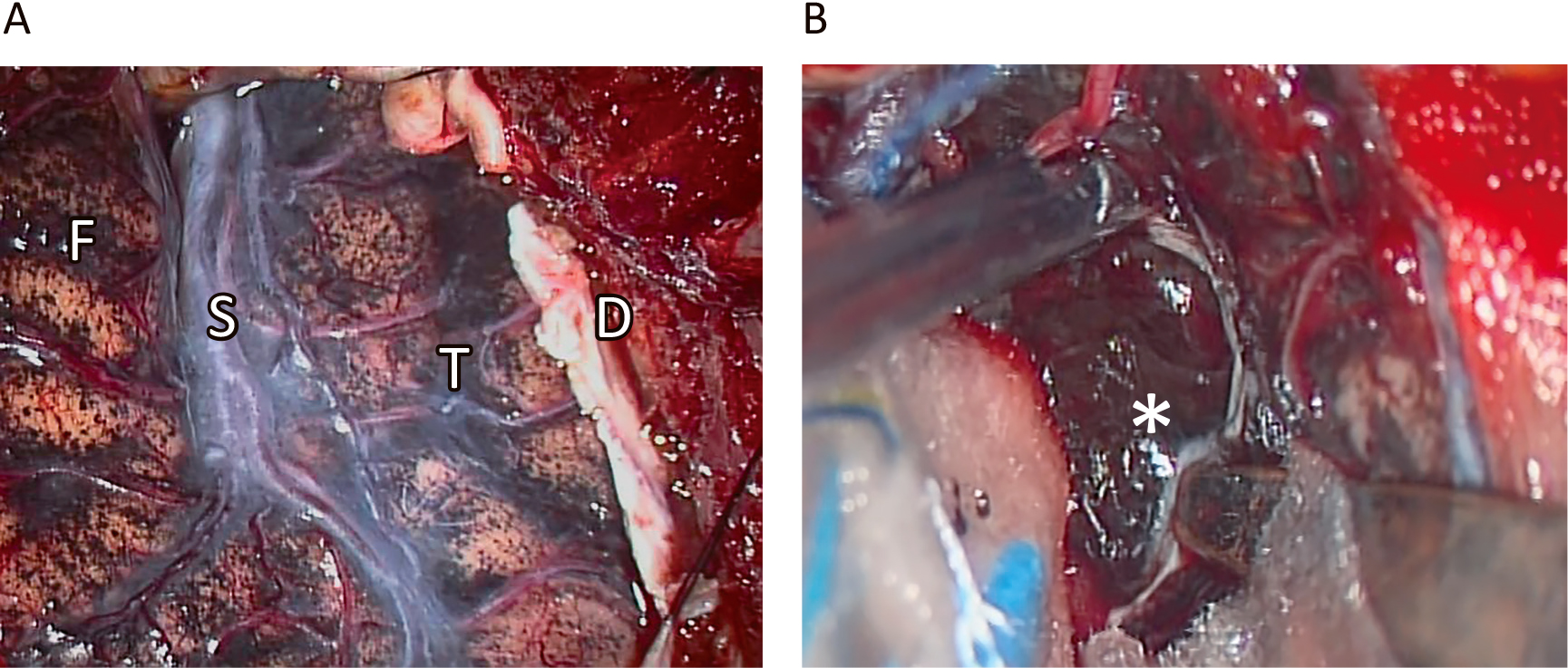
Intraoperative observations. Brain surface was covered by abundant black spots suggestive of subarachnoid dissemination of melanoma (A). Exposure of uncal tumour through Sylvian fissure revealed pitch-black surface interlaced with thin haemorrhage (B). F: Frontal lobe, S: Sylvian fissure, T: temporal lobe, *: tumour, D: dura mater
Haematoxylin-eosin (HE) staining of a surgical specimen showed sheet-like proliferation of heavily pigmented cells with significant pleomorphism, nuclear enlargement, increased nuclear chromatin, and nucleolar prominence (Figure 6A). Immunohistochemically, the cells were positive for HMB-45 (Figure 6B) and melan-A (Figure 6C). The MIB 1 index was 17.7% (Figure 6D). These findings were compatible with malignant melanoma. The cells were negative for BRAF mutation.

Histopathology of surgical specimen. Haematoxylin-eosin (HE) staining showed diffuse proliferation of compact neoplastic cells containing iron deposits, significant nuclear pleomorphism, and conspicuous nucleoli (A). Neoplastic cells were strongly positive for HMB-45 (B) and melan-A (C). MIB-1 index was 17.7% (D).
The patient’s postoperative course was uneventful. She subsequently underwent dermatological assessment, funduscopy, whole-body CT, and whole-body FDG-positron emission tomography (FDG-PET). None of these studies revealed evidence of melanoma or malignancy in other parts of the body.
Despite adjuvant therapy comprising whole-brain radiation (40 Gy in 20 fractions) and an anti-PD-1 inhibitor (5 courses of 160 mg of nivolumab at 3-week intervals), the patient gradually lost awareness, and she became delirious by the end of the 4th month postoperative. MRI showed no recurrence of the right temporal tumour, but widespread dissemination in both the supra- and infratentorial subarachnoid space was observed (Figures 7A–D). The patient was transferred to a nursing home where she died at 4.5 months after the operation, i.e., 33 months after the initial MRI study.

Follow-up magnetic resonance imaging (MRI) study performed 4 months after surgery. Gadolinium-enhanced scans showed strong enhancement of subarachnoid space suggesting thick and widespread dissemination (A: axial, B: coronal, C: sagittal image). No local recurrence was seen (A and D: T2 axial)
In addition to our patient, our literature review found 45 previous patients with primary CNS malignant melanoma (Tables 1A, B)2–5,7–11,13–19,21–25,27–32,35–38).
| Variable | Number of patients | |
|---|---|---|
| Age | ||
| median, mean ± SD | 49, 47.0 ± 18.3 | |
| <40 | 17 (37.0%) | |
| 40–60 | 16 (34.8%) | |
| >60 | 13 (28.3%) | |
| Sex | ||
| male | 24 (52.2%) | |
| female | 22 (47.8%) | |
| Tumor site SD (double count was allowed) | ||
| frontal lobe | 12 | |
| parietal lobe | 10 | |
| temporal lobe | 9 | |
| occipital lobe | 4 | |
| skull base | 5 | |
| cerebellopontine angle | 4 | |
| pineal region | 4 | |
| cerebral sulci | 3 | |
| dura mater | 2 | |
| pons | 1 | |
| Symptoms (double count was allowed) | ||
| headache | 27 | |
| visual disturbance | 10 | |
| nausea/vomiting | 9 | |
| hemiparesis | 8 | |
| seizure | 7 | |
| hearing disturbance | 5 | |
| numbness | 5 | |
| dizziness | 5 | |
| ataxia | 5 | |
| vertigo | 3 | |
| aphasia | 3 | |
| none | 2 | |
| Other skin lesions | ||
| Ota nevus | 5 | |
| uveal melanoma | 1 | |
| hairy navus (leg) | 1 | |
| congenital melanocytic nevus (trunk) | 4 | |
SD (standard deviation)
| Variable | Number of patients | |
|---|---|---|
| Image diagnosis | ||
| T1 high | 36 | |
| T2 low | 16 | |
| strong enhancement | 27 | |
| tumoral hemorrhage | 7 | |
| meningeal dissemination | 8 | |
| Extent of surgical removal | ||
| total excision | 28 | |
| subtotal excision | 8 | |
| unknown | 3 | |
| not performed | 6 | |
| Adjuvant treatment | ||
| radiotherapy | 31 | |
| chemotherapy | 18 | |
| immunotherapy | 3 | |
| Survival | ||
| median survival | 31 months | |
| <1 years | 10 | |
| 1–5 years | 5 | |
| >5 years | 4 | |
T1 high: high intensity on T1 weighted magnetic resonance imaging
T2 low: low intensity on T2 weighted magnetic resonance imaging
Primary CNS malignant melanoma is very rare; the reported incidence is 0.005 cases per 16) individuals26). In the present case, the lesion was incidentally found in a patient who harboured an Ota nevus on the side ipsilateral to the lesion; it disseminated widely over the course of two years.
On MRI scans, CNS melanoma is typically depicted as a round mass hyperintense on T1WI and hypointense on T2WI scans owing to melanin deposits in neoplastic cells. However, these characteristics depend on the concentration of melanin; when fewer than 10% of neoplastic cells are melanin-positive, T1WI may reveal hypointensity and T2WI hyperintensity2,35).
T1WI revealed hyperintensity in our patient. However, as tumour growth was very slow, a diagnosis of cavernous angioma was made in an outside institution, and her referral to us was delayed. Spontaneous haemorrhage is frequently reported in patients harbouring melanoma because the tumour is rich in blood vessels and can easily invade the vessels of the meninges24). Depending on the time course after intratumoural haematoma, intracerebral cavernous angioma may be also hyperintense on T1WI scans. However, on MRI scans, cavernous angiomas tend to display different levels of intensity among multiple cysts, yielding a characteristic “popcorn” or “raspberry” appearance surrounded by a rim of signal loss due to haemosiderin deposits12).
In patients with malignant melanoma, leptomeningeal infiltration by malignant cells is common. The condition is recognized as widespread enhancement of the subarachnoid space and sulci21) and tends to appear in the advanced stage of the disease. In our patient, however, it appeared at the asymptomatic stage.
Primary CNS malignant melanoma is usually aggressive, and approximately 50% of patients previously reported to have the disease died within 31 months. Initially the tumour in our case grew very slowly. Some patients manifest distant metastasis after a quite lengthy interval following the resection of a malignant melanoma. This dormancy may reflect a lack of sufficient tumour vascularity and similar rates of cell proliferation and apoptosis6).
The patient in this case study harboured Ota nevus (oculodermal melanocytosis) around the right eye. Previously, there were reports of 4 patients with Ota nevus; in 3 of these, as in our patient, the lesion was ipsilateral to the intracranial lesion. Ota nevi consist of an extensive patch-like area of dermal melanocytic hyperpigmentation that usually involves the distribution of the first and second branches of the trigeminal nerve. Their embryologic origin is similar to that of intracranial melanocytic lesions. Melanoblasts arise in the neural crest and differentiate into melanocytes that migrate into the skin, ocular structure, and pia mater predominantly on the brain stem and upper cervical spine. Mismigration and neoplastic transformation of these melanocytes may lead to Ota nevus and CNS melanoma11).
In the absence of standardised treatment for malignant CNS melanoma, first-line treatment consists of maximal safe removal followed by external beam radiation (40 to 70 Gy) and chemotherapy26). Methotrexate, dacarbazine, vincristine, temozolomide, and interferon are major chemotherapeutic agents. Anti-PD-1 inhibitors have been used to address malignant melanoma, but their effectiveness in patients with malignant CNS melanoma remains to be established20). After the discovery of the BRAF mutation in malignant melanoma, anti-BRAF agents have been used for malignant melanoma in skin regions. Our literature review found only one report on the use of a BRAF inhibitor in a patient with primary CNS melanoma arising in the pineal gland; however, this treatment was not successful16). Others1,34) have suggested the usefulness of combinations of immunocheckpoint inhibitors, BRAF/MEK inhibitors, and stereotactic radiosurgery for CNS metastases from melanoma. These combinations may be also useful to treat primary CNS malignant melanoma.
According to our literature review, the prognosis of patients with CNS malignant melanoma varied widely; 10 of 27 patients died within 12 months and 4 survived longer than 5 years, including one patient who died 17 years after the initial surgery23). Kaplan-Meier analysis of the overall survival is shown in Figure 8; the median survival time was 31 months. However, once the neoplastic cells spread to the leptomeninges the prognosis is ominous and the survival tends to be 10 weeks33).

Kaplan-Meier analysis of overall survival of 45 previously reported cases and our patient with primary CNS malignant melanoma. Median survival was 31 months.
The present report represents the second reported case of an incidentally found primary CNS malignant melanoma, whose slow growth resulted in a misdiagnosis of cavernous angioma. We suspect that the number of patients with asymptomatic malignant CNS melanoma will rise with the number of MRI studies performed in “healthy” individuals and patients with minor symptoms. Education of local medical care providers regarding the radiologic features of malignant melanoma may result in early diagnosis. To improve outcomes, an effective treatment strategy for primary CNS malignant melanoma is needed.
Ethical ConsiderationAll authors certify that this study was conducted in accordance with the Helsinki declaration (revised in 2000) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (effective February 9, 2015) as ordered by the Ministry of Health, Labor and Welfare, Japan.
Conflict of Interest DisclosureWe declare that each author’s participation in the work was sufficient for taking public responsibility for the content of this manuscript and that no author had a conflict of interest that could be perceived as prejudicial regarding its content.