2013 Volume 53 Issue 10 Pages 1725-1731
2013 Volume 53 Issue 10 Pages 1725-1731
The present study aims to investigate the possibility of reduction of radiative heat flux in the continuous casting process by valence control of iron ions in mould flux. The compositions of samples were designed on the basis of a practical mould flux, where the basicity defined as CaO/SiO2 was also varied from 0.6 to 1.4 to change the valence of iron ions. Mixtures of oxide and fluoride powders were melted in platinum crucibles in air and then the melts were quenched into brass moulds to obtain glassy samples. In addition, graphite crucibles were also used to melt samples having the basicity of 1 at lower partial pressure of oxygen. Some glassy samples were heat-treated for crystallisation. Glassy and crystallised samples were subjected to chemical analyses and optical measurements of apparent reflectivity and transmissivity. The concentration ratio of Fe3+/Fe2+ increased with increasing basicity but decreased by melting in graphite crucibles. Increasing concentration ratio of Fe3+/Fe2+ leads to an increase of radiative heat flux for the glassy samples but to a decrease for the crystallised samples: the effect of valences of iron ions is more prominent in the glassy samples. In the crystallised samples, on the contrary, the degree of crystallinity affects radiative heat flux more strongly than the valence of iron ions.
In the continuous casting process, mould flux plays important roles such as lubrication between the copper mould and the steel shell and protection of the steel surface from re-oxidation. Another role of mould flux is to provide proper heat extraction from the steel to the mould, and is more important in high speed continuous casting which is required for improvement of steel productivity. However, too fast casting may bring about surface defects called “longitudinal cracking” due to thermal stress in the steel shell induced by inhomogeneous solidification.1,2) To avoid such a situation, mild cooling is required because it is effective for improving homogeneity.3)
From the viewpoint of mild cooling by mould flux modification, many studies have been made and reviewed by Mills and Fox:4) Namely, crystallisation of mould flux leads to heat transfer reduction across the mould flux from the steel shell to the mould,5,6) where heat is transferred by both thermal conduction and radiation, the former contributing to the greater part of heat flux.7,8,9) The magnitude of conductive heat flux is affected by thermal conductivity of mould flux, which usually increases by crystallisation.10) Thus, if only thermal conductivity of mould flux were changed by crystallisation, the heat flux would increase. In actuality, however, the heat flux has been found to be reduced by crystallisation. This finding has been explained by the following two mechanisms. First, crystallisation enlarges an air gap layer at the interface between the mould flux and the mould, which enhances the interfacial thermal resistance.6,11,12,13) Second, crystallisation increases the reflectivity and lowers the transmissivity of mould flux, which reduces radiative heat flux.8,14,15) With respect to the radiative heat transfer reduction, Nakada et al.16) have proposed a heat transfer model including the optical process, in which model it is assumed that the crystallised layer reflects the light emitted from the steel shell and return it back to the steel again. Further progress has been made by the present authors,17) who have attempted mild cooling by controlling the crystalline grain size precipitated in mould flux, which has also been suggested by Diao et al. for mould flux containing MnO.18) The present authors have also attempted by removing iron oxides from mould flux19) because such oxides absorb the light and cause re-radiation to the mould, bringing about an increase in radiative heat transfer. Combination of both effects would produce even milder cooling; however, even if iron oxide free mould flux is placed into the mould, iron oxides would inevitably form in the mould flux in coexistence with molten steel during continuous casting20) unless there are additions of reducing agents such as carbon and calcium silicide.
Hence, the present work focuses on valence control of iron ions to decrease the absorptivity of mould flux. Now consider the wavelength range relevant to continuous casting temperatures. Wien’s displacement law says that black body at the melting point of iron emits strongest radiations at wavelengths centered at 1600 nm, which radiations are predominantly absorbed by Fe2+,7) whereas Fe3+ absorbs radiations efficiently below 500 nm in wavelength.7) Thus, the change from Fe2+ to Fe3+ would decrease the absorptivity of mould flux in the near infrared region and instead increase the reflectivity especially for crystallised fluxes whose transmissivity is roughly zero, possibly leading to radiative heat transfer reduction. The redox reaction between Fe2+ and Fe3+ in mould flux is governed thermodynamically by Eq. (1).21)
| (1) |
To investigate the dependencies of apparent reflectivity and transmissivity on the valence of iron ions in mould fluxes, samples were prepared according to chemical compositions of a practical mould flux, as given in Table 1, where T.CaO/SiO2 represents basicity including CaO originating from CaF2 and ranges from 0.6 to 1.4. The samples were synthesized from reagent grade chemicals of SiO2, Al2O3, MgO, Na2CO3, CaF2, Fe2O3 and CaCO3, the last being decomposed into CaO by heating at 1323 K for 43.2 ks. Mixtures of chemicals were melted in platinum crucibles in air at a temperature of 1673 K for 300 s, and then the melts were poured into brass moulds held at 673 K to obtain glassy samples about 5 mm thick. To investigate the effect of partial pressure of oxygen during sample melting, only two samples having the basicity of 1 were also melted in graphite crucibles in argon at a temperature of 1673 K for 300 s. Some of these glassy samples were heat-treated at 933 K for 1800 s to produce crystallised samples. The temperature for crystallisation was determined from an exothermic peak measured by differential scanning calorimetry (DSC).
| T. CaO/SiO2 | CaO | SiO2 | Al2O3+MgO | Na2O+F | Fe2O3 |
|---|---|---|---|---|---|
| 0.6 | 28.4 | 47.4 | 3.3 | 19.8 | 1.0 |
| 0.8 | 33.7 | 42.1 | |||
| 1.0 | 37.9 | 37.9 | |||
| 1.2 | 41.3 | 34.5 | |||
| 1.4 | 44.2 | 31.6 |
X-ray diffraction (XRD) analysis was used to identify the crystallised phase, which was cuspidine (3CaO·2SiO2·CaF2), and to determine the degree of crystallinity of mould fluxes by the internal standard method,10) which is based upon comparison between the intensities of diffraction peaks of cuspidine in the sample and in the reference.
The concentration of Fe2+ was determined by the titration method with potassium di-chromate, and the total concentration of both Fe2+ and Fe3+ was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES).
The apparent reflectivity (Ra) and transmissivity (Ta) of samples were measured in air at room temperature using a spectrophotometer with an integrating sphere covering the wavelength range 300–2600 nm. The presence of scattered light usually causes a serious problem in reflectivity and transmissivity measurements on polycrystalline samples; in the present work, however, the installation of an integrating sphere enabled all scattered light to be collected and detected.
Figure 1 shows the appearance of samples melted in platinum crucibles in air, where the upper are for glassy samples and the lower are for crystallised samples. It is noted that the glassy sample having the basicity of 0.6 is murky and seems partially crystallised - this would be because this sample could not be quenched as quickly as other samples due to its higher viscosity. As the basicity increases, the colour of glassy samples changes from blue-green to tannish, which indicates that Fe2+ changes to Fe3+ because it is known that Fe2+ strongly absorbs the electromagnetic wave at wavelengths centered at 1000 nm; in contrast, Fe3+ does below 500 nm.7) This valence change in iron ions is consistent with the consideration on the basis of Eq. (1). On the other hand, the crystallised samples show milky white colour due to the presence of crystalline grains.

Appearance of glassy and crystallised samples melted in platinum crucibles.
Figures 2(a) and 2(b) show the appearance of glassy and crystallised samples melted in graphite crucibles. The glassy sample exhibits strong blue colour, indicating that this sample contains more Fe2+ than the corresponding sample in Fig. 1 since melting in a graphite crucible provides a more reducing condition. The crystallised sample in Fig. 2(b) shows milky white colour, similarly to the crystallised samples in Fig. 1.

Appearance of glassy and crystallised samples having basicity of 1 melted in graphite crucibles.
Table 2 shows the concentration variation of Fe2+ in the samples associated with the basicity change together with the total iron concentrations. The total concentrations of iron ions for the samples melted in platinum crucibles range roughly from 0.54 to 0.59 mass%, and are slightly smaller than a value of 0.7 mass% which is calculated from a value of 1 mass% of Fe2O3 (the initial amount added to the sample). It can also be seen that the concentration of Fe2+ decreases with increasing basicity, except for the samples having the basicity of 0.6. This would be due to the change of the dominant redox reaction by lowering basicity to a large extent from Eq. (1) to reaction Fe3+ +1/2O2– = Fe2+ + 1/4O2(g), for example. On the other hand, the total concentrations of iron ions for the samples melted in graphite crucibles show even lower values, suggesting that part of iron formed by carbon reduction was removed from the samples. Figure 3 shows the relationship between the basicity of T.CaO/SiO2 and the logarithm of concentration ratio of Fe3+/Fe2+ (ln(Fe3+/Fe2+)) for all the samples, where the concentration of Fe3+ has been calculated by subtracting the concentration of Fe2+ from the total iron concentration in Table 2. The value of ln(Fe3+/Fe2+) for the samples melted in platinum crucibles increases with increasing basicity, as can be expected from the redox reaction of Eq. (1). In addition, the values greatly decrease for the samples melted in graphite crucibles, which condition provides strong reducing atmosphere.
| Glassy | Crucible | T. CaO/SiO2 | Fe2+ | T. Fe |
| Pt | 0.6 | 0.0893 | 0.587 | |
| 0.8 | 0.106 | 0.564 | ||
| 1.0 | 0.0838 | 0.550 | ||
| 1.2 | 0.0670 | 0.652 | ||
| 1.4 | 0.0558 | 0.586 | ||
| C | 1.0 | 0.402 | 0.493 |
| Crystallised | Crucible | T. CaO/SiO2 | Fe2+ | T. Fe |
| Pt | 0.6 | 0.0664 | 0.563 | |
| 0.8 | 0.136 | 0.561 | ||
| 1.0 | 0.101 | 0.567 | ||
| 1.2 | 0.0876 | 0.549 | ||
| 1.4 | 0.0531 | 0.537 | ||
| C | 1.0 | 0.428 | 0.492 |

Relationship between basicity of T. CaO/SiO2 and logarithm of concentration ratio of Fe3+/Fe2+ for all samples.
Figure 4 shows the change in the degree of crystallinity with the basicity for the crystallised samples melted in platinum crucibles and a graphite crucible. The degree of crystallinity increases almost in linear proportion to the basicity. In addition, comparison between the two samples having the basicity of 1 also indicates that the degree of crystallinity is not affected considerably by the melting conditions, from the fact that experimental scatter in the degree of crystallinity is typically within ± 5%.17) Thus, the difference between experimental results for these two samples would be responsible for the difference in the concentration ratio of Fe3+/Fe2+.

Change in degree of crystallinity with basicity for all crystallised samples.
Figures 5(a) and 5(b) show apparent reflectivities (Ra) and transmissivities (Ta) for the glassy samples melted in platinum crucibles as functions of wavelength, and Fig. 5(c) shows corresponding apparent absorptivities (Aa) derived from the following equation.
| (2) |
| (3) |

(a) Apparent reflectivities, (b) transmissivities and (c) absorptivities of glassy samples melted in platinum crucibles.
Figures 6(a) and 6(b) show apparent reflectivities and transmissivities for the crystallised samples melted in platinum crucibles as functions of wavelength, and Fig. 6(c) shows corresponding apparent absorptivities. With increasing basicity, the reflectivity increases around 1000 nm, whereas the transmissivity is almost zero because of polycrystalline samples; resultantly, the absorptivity decreases complementarily to the reflectivity. These spectra changes by the basicity are not the same as those in Fig. 5. Thus, the spectra for the crystallised samples would be affected by crystallisation itself rather than the concentration ratio of Fe3+/Fe2+ controlled by the basicity.

(a) Apparent reflectivities, (b) transmissivities and (c) absorptivities of crystallised samples melted in platinum crucibles.
Figures 7(a) and 7(b) show apparent reflectivities and transmissivities for the glassy sample having the basicity of 1 melted in a graphite crucible as functions of wavelength, in comparison with those for the glassy sample melted in a platinum crucible with the same basicity, and Fig. 7(c) shows corresponding apparent absorptivities. The reflectivity remains roughly zero even if the concentration of Fe2+ significantly increases. In contrast, the transmissivity of the sample melted in a graphite crucible is on the whole much smaller than that of the sample melted in a platinum crucible, and the absorptivity is complementary to the transmissivity.
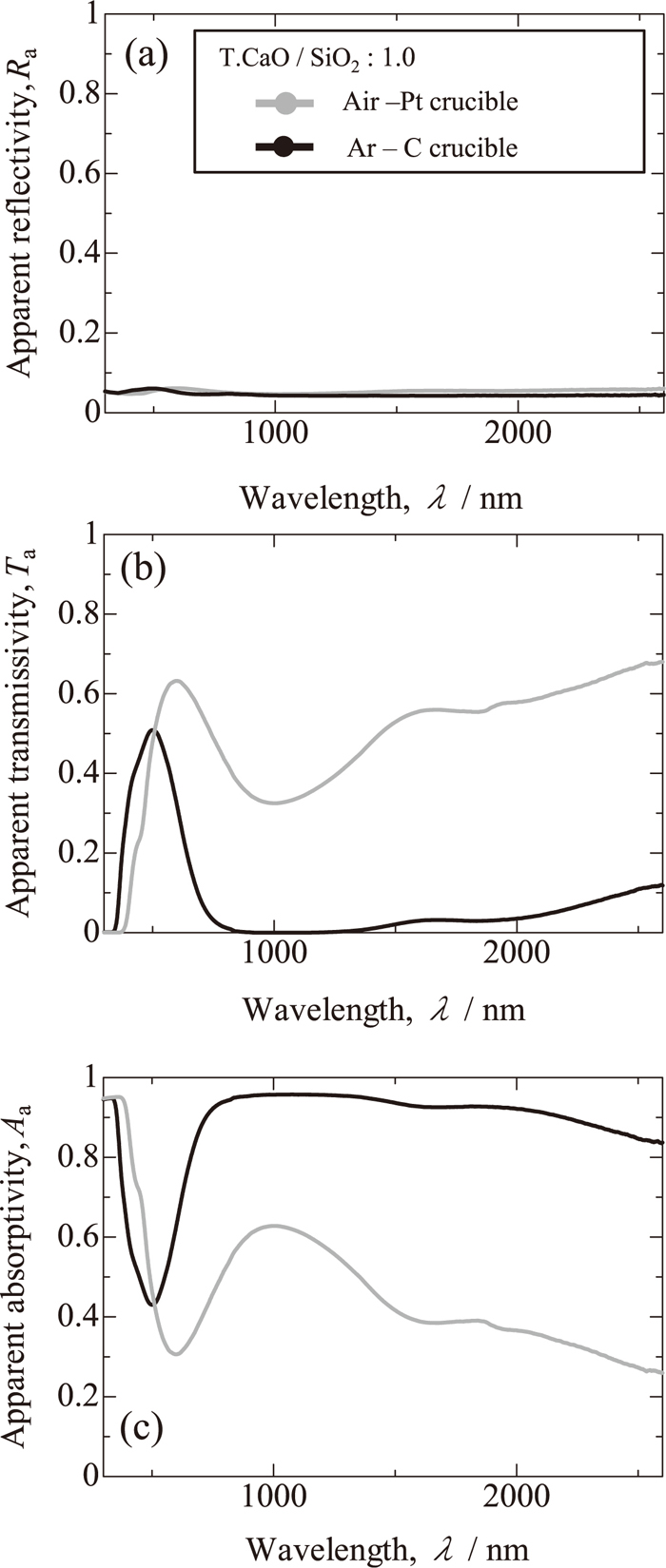
(a) Apparent reflectivities, (b) transmissivities and (c) absorptivities of glassy sample having basicity of 1 melted in graphite crucible.
Figures 8(a) and 8(b) show apparent reflectivities and transmissivities for the crystallised sample having the basicity of 1 melted in a graphite crucible as functions of wavelength, in comparison with those for the crystallised sample melted in a platinum crucible with the same basicity, and Fig. 8(c) shows corresponding apparent absorptivities. The reflectivities for both samples are increased due to crystallisation, and the sample melted in more reducing atmosphere gives smaller reflectivities than the other, which is because Fe2+ shows stronger absorption than Fe3+ in the near infrared region, as shown in Fig. 8(c). As a result, the transmissivities are close to zero almost all over the wavelength range measured.

(a) Apparent reflectivities, (b) transmissivities and (c) absorptivities of crystallised sample having basicity of 1 melted in graphite crucible.
The optical-process model previously proposed by three of the authors17,19) is applied to examine the effect of basicity on radiative heat transfer across mould flux. This model assumes that mould flux film between the steel shell and the mould consists of liquid and solid layers - the liquid layer is next to the steel and the solid layer is next to the mould. It is also assumed that there is no absorption by the liquid layer but there is multi-reflection within the liquid layer. Consequently, this model gives the total radiative heat flux which may reach the mould (ITotal) as
| (4) |
Figure 9 shows the change in the total radiative heat flux with the basicity for the glassy and crystallised samples melted in platinum crucibles. With increasing basicity, the total radiative heat flux increases in the glassy samples. As shown in Figs. 5(b) and 7(b), for the glassy samples, the transmissivity increases in the near infrared region with increasing basicity via an increase in the concentration ratio of Fe3+/Fe2+, which would lead to an increase in the total radiative heat flux according to Eq. (4). For the crystallised samples, in contrast, the value of ITotal decreases by about 30% with increasing basicity from 0.6 to 1.4, which suggests that increasing basicity is effective for radiative heat transfer reduction; however, the basicity change may cause to change the degree of crystallinity as well as the concentration ratio of Fe3+/Fe2+. Thus, it is impossible to understand the change of ITotal with the basicity only from the viewpoint of the concentration ratio of Fe3+/Fe2+ at this stage. The following discusses which contribution is more dominant.

Change in total radiative heat flux with basicity for glassy and crystallised samples melted in platinum crucibles.
Figure 10 re-plots data given in Figs. 4 and 9, and shows the effect of the degree of crystallinity on the total radiative heat flux for the crystallised samples melted in platinum crucibles. The total radiative heat flux seems affected by the degree of crystallinity as well only from this figure, which is examined in more details. Figure 11 shows the relationship between ln(Fe3+/Fe2+) and ITotal values for both glassy and crystallised samples using Fig. 3 as well, where values of the degree of crystallinity are stated only for the crystallised samples. For the glassy samples, the total radiative heat flux increases with increasing concentration ratio of Fe3+/Fe2+ and would be dominated by transmissivity increase in Eq. (3), as mentioned before.

Effect of degree of crystallinity on total radiative heat flux for the crystallised samples melted in platinum crucibles.

Relationship between ln(Fe3+/Fe2+) and ITotal values for both glassy and crystallised samples.
For the crystallised samples, however, the total radiative heat flux decreases with increasing concentration ratio of Fe3+/Fe2+, from comparison between data for the crystallised samples having almost the same degree of crystallinity, say 50%. This can be understood as follows. As shown in Figs. 6 and 8, the transmissivity is close to zero for the crystallised samples and the reflectivity and absorptivity are complementary. The increase in the concentration ratio of Fe3+/Fe2+ leads to an increase of the reflectivity and to a decrease of the absorptivity in the near infrared region. The latter would dominate the reduction of total radiative heat flux according to Eq. (4).
Compare the slopes of the straight lines for the glassy samples and crystallised samples having a degree of crystallinity of 50%. It can be seen that the magnitude of the slope for the glassy samples is greater than that for the crystallised samples. Thus, the concentration ratio of Fe3+/Fe2+ gives a stronger effect to the radiative heat flux in glassy fluxes. Now focus on ITotal values for the crystallised samples melted in platinum crucibles. With increasing concentration ratio of Fe3+/Fe2+, the ITotal value decreases to a larger extent although the change in the ratio is smaller in comparison with the crystallised samples with a degree of crystallinity of 50%. Accordingly, the decrease in the ITotal value for the crystallised samples melted in platinum crucibles would be dominated by the degree of crystallinity rather than the concentration ratio of Fe3+/Fe2+. Mould fluxes used are usually crystallised in actual operations, and thus the valence control of iron ions in mould fluxes is effective but not a very efficient measure for radiative heat flux reduction.
Glassy and crystallised mould flux samples with different concentration ratios of Fe3+/Fe2+ have been prepared by changing the basicity of samples and the atmosphere for melting. The apparent reflectivity and transmissivity have been measured to evaluate radiative heat fluxes.
• For the glassy samples, the reflectivity is as low as 5%, irrespective of the concentration ratio of Fe3+/Fe2+, and the transmissivity and absorptivity are complementary. The transmissivity increases with increasing concentration ratio of Fe3+/Fe2+ in the near infrared region, which would lead to an increase in the total radiative heat flux.
• For the crystallised samples, the transmissivity is almost close to zero, irrespective of the concentration ratio of Fe3+/Fe2+, and the reflectivity and absorptivity are complementary. The absorptivity decreases with increasing concentration ratio of Fe3+/Fe2+ in the near infrared region, which would lead to a decrease in the total radiative heat flux, namely mild cooling.
• The degree of crystallinity plays a more important role than the valence of iron ions from the perspective of radiative heat transfer reduction in crystallised mould fluxes.