2013 Volume 53 Issue 12 Pages 2167-2175
2013 Volume 53 Issue 12 Pages 2167-2175
The effect of Mg and Ti addition on the solidification macrostructure of ferritic stainless steel was evaluated in order to obtain fine equiaxed grains. Equiaxed solidification was promoted by adding Mg and Ti. Ti addition affects the amount of TiN as a nucleation agent of δ-Fe and the increase in constitutional undercooling. Mg addition produces spinel oxide in the molten steel, which accelerates the TiN formation. [Al] content also have an influence on the equiaxed grain formation.
For ferritic stainless steel, it is an important technical challenge to suppress the occurrence of ridging.1,2) Ridging is a surface roughness that occurs when metal products are fabricated. In general, ridging causes disfigurement of the surface and a grinding process is required for amelioration. Ridging is partly caused by the large columnar structures formed during solidification. Because phase transformation from austenite (γ) to ferrite (α) does not occur in high-purity ferritic stainless steel, we cannot expect a fine-grained structure transformed from a coarse-grained structure, and the cast slab generally exhibits a large columnar macrostructure after solidification and cooling. Ridging is usually avoided by stimulating recrystallization during hot rolling or repeated cycles of cold rolling and annealing. Moreover, recrystallization is promoted if fine-grained or equiaxed macrostructures are formed, and consequently, the ridging characteristics can be improved.3)
In order to form fine-grained or equiaxed macrostructures, some methods have been employed, such as low superheat casting, electromagnetic stirring, and the use of inoculants.4,5,6) However, these methods have not yielded successful results for high-purity ferritic stainless steel. TiN, which shows good coherency of crystal lattice parameters with ferrite phase, has been usually used as a nucleating agent.6,7,8) Koseki et al. observed the formation of ferrite by using TiN crystal nuclei in their liquid-tin quenching measurements.8) They further showed that the regions where equiaxed grain structures formed approximately coincided with the region where the crystallization of TiN occurred at the liquidus temperature predicted by equilibrium calculation. In addition, they analyzed heterogeneous nucleation in the constitutional undercooling region.8) Furthermore, to accelerate the formation of fine-grained equiaxed structures, uniformly dispersed spinel (Al2MgO4) in molten steel, which act as nucleation sites to crystallize TiN, have been used.9) It is claimed that spinel oxides and TiN show good crystallographic consistency, although the lattice consistency has not been examined and the equiaxed structure formation has also not been analyzed. In addition, the formation of fine-grained equiaxed macrostructures is also reported to be accelerated by the addition of Mg in carbon steel,10) and it is considered that MgO or spinel could act as heterogeneous nucleation sites for ferrite, although much less effectively than TiN. However, by adding Mg and Ti into 16%Cr steel, the columnar structures are reported to become fine grained, and equiaxed grain structures appear hard to obtain, considering that constitutional undercooling is small because of the small amount of alloying composition.11) In this study, the influence of the addition of Mg and Ti in 11%Cr steel was investigated with the aim of obtaining fine-grained equiaxed macrostructures in high-purity ferritic stainless steel. Our study revealed interesting information on the formation of equiaxed structures.
Table 1 shows the chemical composition of the samples used in this study. The basic component of the test material was 11%Cr–0.006%N steel and samples comprising six different amounts of Ti with or without Mg or six different amounts of Al were chosen for the study (the total number of samples was 18). Although the Ti content was widely varied, the calculation with regard to TiN formation at the liquidus temperature using Thermo-Calc12) showed that at the compositions we used (the composition was fixed to that of sample No. 1, with the exception of the amounts of Ti and N), TiN could be formed at temperatures above the liquidus temperature in sample Nos. 5, 6, 11, and 12. It is known that the formation of equiaxed structures is accelerated by specific compositions in which the Ti and N contents are above the TiN crystallization curve at the liquidus temperature,8) and it can be assumed that sample Nos. 5, 6, 11, and 12 satisfy these conditions. The samples were collected from the ingot cast in a 50-kg vacuum induction melting furnace. In sample Nos. 1–13, Al was added only during the early deoxidation stage of melting, and then Ti was added. The melts were prepared at 1600 ± 20°C (the superheat was set to about 90°C), after which the materials were tapped into the mold. In the samples Nos. 14–18, Al was added prior to Ti (attention is drawn to the order of addition) before tapping. Mg was placed in the mold and was added in the samples by pouring the molten metal onto it.
| C | Si | Mn | P | S | Cr | Ti | Al | Mg | T.O | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0057 | 0.40 | 0.40 | 0.021 | 0.007 | 11.0 | 0.012 | 0.010 | – | 0.0042 | 0.0060 |
| 2 | 0.0056 | 0.40 | 0.40 | 0.020 | 0.006 | 11.0 | 0.051 | 0.010 | – | 0.0036 | 0.0059 |
| 3 | 0.0058 | 0.40 | 0.40 | 0.021 | 0.006 | 11.0 | 0.100 | 0.010 | – | 0.0033 | 0.0058 |
| 4 | 0.0056 | 0.40 | 0.40 | 0.021 | 0.006 | 11.0 | 0.200 | 0.011 | – | 0.0033 | 0.0060 |
| 5 | 0.0051 | 0.40 | 0.40 | 0.020 | 0.006 | 11.0 | 0.396 | 0.013 | – | 0.0029 | 0.0057 |
| 6 | 0.0054 | 0.40 | 0.40 | 0.021 | 0.006 | 11.0 | 0.583 | 0.013 | – | 0.0025 | 0.0060 |
| 7 | 0.0053 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.013 | 0.005 | 0.0009 | 0.0036 | 0.0055 |
| 8 | 0.0053 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.051 | 0.005 | 0.0006 | 0.0036 | 0.0056 |
| 9 | 0.0052 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.097 | 0.007 | 0.0005 | 0.0036 | 0.0056 |
| 10 | 0.0048 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.186 | 0.010 | 0.0008 | 0.0034 | 0.0056 |
| 11 | 0.0053 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.361 | 0.015 | 0.0014 | 0.0034 | 0.0056 |
| 12 | 0.0055 | 0.40 | 0.41 | 0.020 | 0.006 | 10.9 | 0.520 | 0.020 | 0.0015 | 0.0034 | 0.0056 |
| 13 | 0.0049 | 0.41 | 0.41 | 0.021 | 0.006 | 11.0 | 0.190 | 0.012 | 0.0008 | 0.0027 | 0.0056 |
| 14 | 0.0046 | 0.41 | 0.41 | 0.021 | 0.006 | 11.1 | 0.200 | 0.018 | 0.0009 | 0.0027 | 0.0058 |
| 15 | 0.0046 | 0.41 | 0.41 | 0.021 | 0.006 | 11.1 | 0.200 | 0.036 | 0.0012 | 0.0023 | 0.0058 |
| 16 | 0.0045 | 0.41 | 0.40 | 0.021 | 0.006 | 11.0 | 0.190 | 0.055 | 0.0012 | 0.0022 | 0.0060 |
| 17 | 0.0052 | 0.41 | 0.41 | 0.021 | 0.006 | 11.1 | 0.200 | 0.075 | 0.0023 | 0.0019 | 0.0059 |
| 18 | 0.0045 | 0.41 | 0.41 | 0.021 | 0.006 | 11.1 | 0.200 | 0.095 | 0.0009 | 0.0018 | 0.0060 |
The macrostructure of each ingot was investigated at the center of the sample in the longitudinal direction, and the equiaxed crystal ratio and grain sizes were measured. In addition, the distribution of Ti-rich inclusions was also determined using an electron probe microanalyzer (EPMA). EPMA measurement was conducted with a pitch of 1 μm in an area of 500 μm × 500 μm, and the existence of Ti-rich inclusions was defined by the average Ti intensity of +5σ or higher. Furthermore, using selective potentiostatic etching by electrolytic dissolution (SPEED) method, we collected the inclusions from sample No. 10 and observed it by transmission electron microscopy (TEM).
2.2. Liquid-tin Quenching ExperimentsQuench tests8) were conducted using molten tin on two types of samples (Nos. 4 and 10), i.e., 11%Cr–0.2%Ti–0.006%N steel and the sample containing Mg. The structure was frozen by pouring molten tin at about 300°C during TIG welding (performed under the conditions of 150 A–12 V–10 cm/min), and the structure of the solid/liquid interface region was observed with an optical microscope. In addition, the structure and inclusions were also observed by a scanning electron microscope (SEM). Moreover, the inclusions found at the center of an equiaxed dendrite were sliced by a focused ion beam (FIB) method, and the composition and structure of the inclusions were analyzed by TEM and energy dispersive X-ray analysis (EDX).
Figure 1 shows the macrostructures of the ingots obtained in the experiment. The equiaxed crystal ratio increased with an increase in the Ti content. Further, assuming the same Ti content, the addition of Mg increased the equiaxed crystal ratio. Figure 2 shows the relationship between the equiaxed crystal ratio and Ti content. As discussed above, the materials containing Mg showed a larger equiaxed crystal ratio than the sample without Mg, and only about 0.1% Ti was required to achieve the equiaxed crystal ratio of 60% in the materials containing Mg, which was four times smaller than that required for samples without Mg (0.4%). Furthermore, the equiaxed crystal ratio was almost 100% in sample Nos. 11 and 12, which contained a large amount of Ti and were doped with Mg. Figure 3 shows the relationship between the columnar grain width and Ti content. At a depth of 10 mm from the surface layer of ingot, the columnar grain width was inversely dependent on the Ti content. In addition, the columnar grain width of the Mg-added material decreased with an increase in Ti content. Moreover, at a depth of 30 mm from the surface layer of ingot, the sizes of the equiaxed grains decreased with an increase in the Ti content, as shown in Fig. 4, especially when Mg was added.

Macrostructures of 50 kg ingots in ferritic stainleess steels.

Effect of Ti and Mg addition on equiaxed zone ratio.

Effect of Ti and Mg addition on columnar grain width.

Effect of Ti and Mg addition on equiaxed grain size.
Figure 5 shows the distribution of Ti-rich inclusions obtained by using EPMA. The distribution density of the Ti-rich inclusions was about 1.5 times larger in the Mg added material (in sample No. 11, the density was 115/mm2) than that in the sample without Mg (in sample No. 5, the density was 80/mm2). Figure 6 shows the TEM image of the inclusions found in sample No. 11; it contained Mg. TiN was observed on the outer areas, whereas Al and Mg were present in the central regions. It implies that TiN may have crystallized around the spinel.

Distribution of TiN inclusions in cast ingots.

TEM image of inclusion in Mg added ingot.
The influence of Al on the macrostructure is shown in Fig. 7. If the Al content was low (as in sample No. 13), fine-grained equiaxed structures with grain sizes of about 1 mm were found with an equiaxed crystal ratio of approximately 80%. As the Al content increased, the grain sizes of the equiaxed structures increased and the equiaxed crystal ratio decreased. Therefore, in high-purity ferritic stainless steel, the equiaxed crystal ratio increased with an increase in Ti content, and the enhancement in the ratio was suppressed if the Al content was high.

Macrostructures of 50 kg ingots varying [Al] content.
Figure 8 compares the liquid-tin quenched structure in the sample containing Mg (sample No. 10) and in that without Mg (sample No. 4). In the Mg-doped material, fine grain structures were formed in the molten pool region near the quenched interface. Figure 9 shows the SEM image of an equiaxed dendrite. The dendrite size was approximately 20 μm, in which inclusions were observed at the central part. Figure 10 shows the results of the FIB-TEM analysis of the inclusions present at the central part of a dendrite. In the inclusions, elliptically shaped Mg2TiO4 was found to surround MgO in the central part and nearly octagonal Al2MgO4 surrounded Mg2TiO4. TiN crystallized in the flat and smooth part of Al2MgO4, whereas the thickness of TiN was as low as around 20 nm. While there were no clear crystallographic relationships between MgO and Mg2TiO4, Mg2TiO4//Al2MgO4 and Al2MgO4//TiN showed good coherency of lattice parameters. However, coherency of the lattice parameters of TiN and ferrite was not observed.

Grain structure of weld solidification quenched by liquid tin.
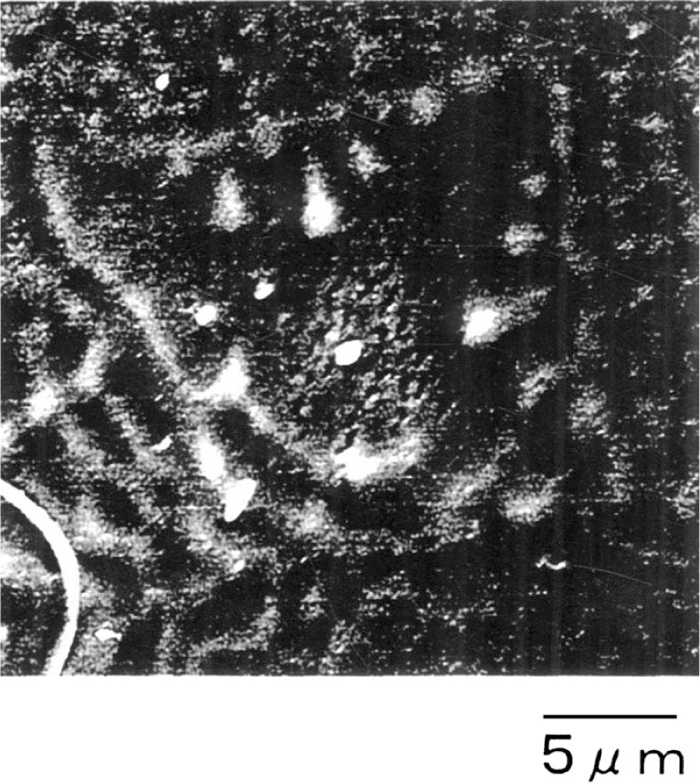
Dendritic structure obtained by liquid tin quenching experiment.

FIB-TEM analysis of inclusion obtained by liquid tin quenching experiment.
To analyze the possibility of equiaxed structures formation, the dendrite tip temperature (T*) was calculated by the KGT (Kurz–Giovanola–Trivedi) model as given by Eq. (1).13,14,15,16) For these calculations, the compositions were assumed to be either Fe–0.005%C–0.40%Si–0.40%Mn–0.02%P–11.0%Cr–0.19% or 0.36%Ti–0.006%N. The temperature gradient was assumed to be 100 K/cm and 10 K/cm corresponding to the values at the surface layer and the intermediate region, respectively, and they were obtained by the heat transfer analysis of bloom continuous casting.
| (1) |
In Eq. (1), TL is the liquidus temperature, mv,i is the slope of liquidus temperature,
| parameter | value | ref. | |
|---|---|---|---|
| liquidus temperature (K) | 1783.75 | 12),18) | |
| liquidus slope (K/mass%) | C | –87 | 12),18) |
| Si | –11 | ||
| Mn | –5.4 | ||
| P | –40 | ||
| Cr | –0.97 | ||
| Ti | –19 | ||
| N | –59 | ||
| partition coefficient | C | 0.19 | 12),18) |
| Si | 0.80 | ||
| Mn | 0.78 | ||
| P | 0.09 | ||
| Cr | 0.97 | ||
| Ti | 0.33 | ||
| N | 0.28 | ||
| diffusion constant (m2/sec) | C | 1.43×10–7 | 19) |
| Si | 2.4×10–8 | ||
| Mn | 1.47×10–6 | ||
| P | 3.1×10–7 | ||
| Cr | 2.67×10–7 | ||
| Ti | 1.81×10–7 | ||
| N | 1.43×10–7 | ||
| activation energy (J/mol) | C | 4.69×104 | 19) |
| Si | 1.72×104 | ||
| Mn | 5.06×104 | ||
| P | 4.60×104 | ||
| Cr | 6.69×104 | ||
| Ti | 4.77×104 | ||
| N | 4.69×104 | ||
| Gibbs-Thomson coefficient (mK) | 2.8×10–7 | 20) | |
As shown in Figs. 11 and 12, the high-purity ferritic stainless steel was characterized by a small undercooling at the dendrite tip. This is because the constitutional undercooling was low due to the partition coefficient of high-concentration Cr taking a value close to unity. In addition, the C and N with small partition coefficients had low concentration in the high-purity ferritic stainless steel. At the solidification velocity of 2 × 10−3 m/s (assuming initial solidification), undercooling was 3–4 K at the dendrite tip, which was greater by about 1 K in sample with high Ti content (Fig. 12).

Calculated dendrite tip temperature.

Calculated dendrite tip temperature.
We can explain the formation of equiaxed structures by the Hunt model21) or the GTK (Gäumann-Trivedi-Kurz) model22) in the constitutional undercooling area ahead of the dendritic solidification front. In this study, the growth of the dendrite was dealt with the GTK model to examine the possibility of the formation of equiaxed structures. Our targets of analysis were the full-scale equiaxed structure formation in sample No. 11 (with a Ti content of 0.36%) and the significantly accelerated equiaxed structure formation in sample No. 10 (with a Ti content of 0.19%). For the calculations, the temperature gradient and solidification velocity in the two cases were assumed to be 100 K/cm and 2 mm/s, and 10 K/cm and 0.2 mm/s, respectively. Figures 13 and 14 show the local temperature profile ahead of the dendritic solid/liquid interface. The difference between the local temperatures calculated from the liquidus temperature profile and temperature gradient shows the constitutional undercooling of the solidification front.

Equiribrium liquidus and local temperature profile ahead of a moving interface for [Ti] = 0.36%. V = 2 mm/s, G = 100 K/cm.
The formation of equiaxed structures in the constitutional undercooling region can be estimated by integrating the crystal growth in accordance with the degree of undercooling up to the dendrite tip. like the GTK model, the degree of undercooling was obtained as the sum of constitutional undercooling, curvature undercooling, and kinetic effect. The grain size of the equiaxed structure (re) is given by Eq. (2).21,22)
| (2) |
| (3) |
| (4) |
| Disregistry (%) | |
|---|---|
| MgO·Al2O3 | 1.4 |
| MgO | 2.8 |
| TiN | 3.9 |
| Ce2O3 | 5.0 |
| Al2O3 | 16.1 |

Equiribrium liquidus and local temperature profile ahead of a moving interface for [Ti] = 0.19%.V = 0.2 mm/s, G = 10 K/cm.
If the critical degree of undercooling for δ-ferrite is assumed to be 1.0 K by TiN, the constitutional undercooling was greater than the critical degree of undercooling in both Figs. 13 and 14, and the formation of equiaxed structures could be possible. Therefore, the result shown in Fig. 15 was obtained when we calculated the volume fraction of the equiaxed structure at the dendrite tip using Eqs. (2), (3), (4) by assuming N0 values of 10–106/cm3. When the Ti content was 0.19% (corresponding to Fig. 14), it is thought that approximately 2500/cm3 (2.5/mm3) nucleation sites were necessary for the formation of equiaxed structures (with the volume fraction of equiaxed grain structures of 0.5 or higher). When the Ti content was 0.36% (corresponding to Fig. 13), approximately 68000/cm3 (68/mm3) nucleation sites were necessary for the formation of equiaxed structures because of higher temperature gradient. The number of spherical inclusions per unit volume (Nv) was obtained from Ns per unit area and the average inclusion size (d) as Nv = Ns/d. Considering the number of TiN distribution in the ingot obtained in this study (about 100/mm2), sufficient number of nucleation sites might be present, although the formation period of TiN was difficult to quantify. In addition, the grain size of TiN was about 1 μm (Fig. 6), and it is thought that this size is large enough for heterogeneous nucleation.

Effect of nucleation site on volume fraction of equiaxed grain.
It is also necessary to explain the grain size (several mm) of the solidified structure, which is different by many orders of magnitude when compared with the number of nucleation sites (dozens/mm3) mentioned earlier. Considering that the steel samples with very few impurities used in this study were composed of single-phase ferrite from solidification until the room temperature, it is thought that the grain growth is remarkably fast in comparison with the other types of ferritic stainless steels, especially in the high temperature region (≧1200°C) soon after solidification. It is further considered that the cooling rate is relatively slow after solidification because we used relatively large steel ingots (of 50 kg). It is therefore suggested that, although the actual grains are relatively small soon after solidification, because ferrite phase was formed finely in accordance with the number of heterogeneous nucleation sites, the grain sizes increased during the cooling process after solidification, making the size of the equiaxed grain structures greater than that expected from the number of heterogeneous nucleation sites. Larger grain sizes due to grain growth were observed in the equiaxed structure at the central part of the ingot where the cooling rate was slower than the surface parts, as shown in sample Nos. 10 and 11 of Fig. 1.
In addition, because of high superheat, the conditions used in this study are not suitable for the formation of equiaxed structures. However, the constitutional undercooling was relatively large, and the formation of equiaxed structures may still have been possible if there were sufficient number of heterogeneous nucleation sites. Thus, it is thought that the addition of Ti contributed to the formation of equiaxed structures through the two factors: the formation of TiN as nucleation sites and the enhancement of constitutional undercooling.
4.2. Role of Mg in the Formation of Equiaxed Grain StructuresRegardless of the presence or absence of Mg, the formation of equiaxed structures was promoted by increasing the Ti content. Equiaxed grain structures were not obtained when the content of Ti was 0.05% or lower, even if Mg was added. In addition, the observation of TiN crystallizing on the Mg-rich inclusions at the center of a dendrite in the liquid-tin quench test suggests that TiN acted as the inoculation nucleus of δ-Fe solidification. With the assumption that the formation of equiaxed structures was associated with the crystallization of TiN at the liquidus temperature,7) we examined the relationship between the solubility product of TiN and the solidification structure. Figure 16 shows the relationship between the solubility product of Ti and N at the liquidus temperature and the formation of equiaxed structures with an equiaxed ratio of above 50%. The crystallization limit of TiN and equiaxed structure formation in the material were correlated even in the absence of Mg, whereas the equiaxed grain structures were formed even below the solubility product of Ti and N in the samples containing Mg. Therefore, in the absence of Mg, the formation of equiaxed structures can be estimated from the equilibrium calculation. However, in the presence of Mg, the formation of equiaxed structures cannot be explained solely by the equilibrium calculation or by the analysis of equiaxed growth using the constitutional undercooling model discussed in the preceding paragraph. Hence, it is considered that the mechanism of the crystallization of TiN is affected by the addition of Mg.

Effect of composition on equiaxed structure formation in 50 kg ingots.
Considering that the heterogeneous nucleation by the spinel was insignificant, the distribution density of inclusions was not markedly enhanced by the addition of Mg, and the influence of the Al content was very large, we pursued the factors other than the creation of heterogeneous nuclei generated by the addition of Mg and formation of spinel.
First, we discuss the reason for the creation of TiN below the solubility product. Figure 17 shows the distribution of Ti content ahead of a moving dendrite corresponding to Fig. 14. The Ti content was about 0.21% even in the solute concentration enriched region near the solid/liquid interface, which is lower than the threshold Ti content (0.30%) required for creating TiN (as predicted by the equilibrium calculation). In addition, if we consider the effect of Mg on the activity of Ti, Mg lowers the activity of Ti,25) and it is difficult to conceive that the addition of Mg could promote the creation of TiN.

Concentration profile ahead of a moving interface. [Ti] = 0.19%, V = 0.2 mm/s, G = 10 K/cm.
Then, we discuss the contribution of the deoxidation reaction to the creation of TiN. It is noted that the inclusions of a complex layered structure were observed (Fig. 10), and the formation of equiaxed structures was promoted at low Al concentrations (Fig. 7).
Figure 18 shows the results of the thermodynamic evaluation,26,27) indicating the influence of Al and Ti content on the oxide composition before the addition of Mg. For this analysis, the composition and temperature were assumed to be 11%Cr–0.006%N–0.005%O–Al–Ti and 1800 K, respectively. The cell model28) was used for the calculation of the activities of the oxide components in the liquid phase, and the pure oxides and more than 20 types of multiple oxides were simultaneously considered in the solid phase. The minimum free energy of the system was evaluated by the equilibrium calculation between the inclusions and molten steel. It was shown that the inclusions changed from (Ti2O3) to (Al2O3) when the Ti content was low or the Al content was high. From the experimental results of different Al contents (Fig. 7) with the same solubility product level of TiN and from Fig. 18, it is further shown that if the inclusions were composed of Ti2O3 before the addition of Mg, equiaxed grain structures were formed (No. 13). If the inclusions were composed of Al2O3 before the addition of Mg, columnar structures were formed (sample Nos. 15–18). From these results, although Ti2O3 formed as a deoxidation product when the Al content was low, it is thought that Mg reduced Ti2O3 and Al2MgO4 formed, and subsequently, the locally enriched Ti allowed the formation of TiN on Al2MgO4. Therefore, the following reactions can be expected to occur at low Al content.
| (5) |
| (6) |

Effect of [Al] and [Ti] content on inclusion composition.
Considering that Mg was added in a low temperature mold in this study, it seems to assume the occurrence of the reactions mentioned above and conclude that the formation of equiaxed grain structures was promoted because the inclusions containing TiN were finely dispersed. As shown in Fig. 10, the presence of TiN at the central part of a dendrite at the early stage of solidification in samples containing Mg and the good coherency observed between TiN and Al2MgO4 suggest that Al2MgO4 created by deoxidation promoted the crystallization of TiN. In addition, the presence of Mg2TiO4 in the inner layer of Al2MgO4 (Fig. 10) also suggests the importance of titanium oxide formation before the addition of Mg.
The grain size of equiaxed structures is assumed to be reciprocally correlated with the number of equiaxed grain structures. The grain size of the equiaxed structures reduced if many solidification nuclei were present. The grain size of the equiaxed structures reduced with an increase in the Ti content because of the increase in the number of crystallized TiN. The refinement of equiaxed structures by Mg addition is also assumed to stem from the creation of Mg-rich inclusions, which act as the nucleation site for TiN. Compared with samples without Mg (assuming the same Ti content), the large effect obtained in samples containing Mg is thought to be attributed to the larger TiN distribution density, as shown in Fig. 5.
Finally, our hypothesis that the formation of TiN is promoted by Mg is compared with the results obtained by Fujimura et al.9) In their study, the spinel was shown to promote the creation of TiN similar to our experiments. However, the process of creating a spinel and TiN are different from each other. While they claimed that the liquid Al–Mg–Ti oxide formed by homogeneous nucleation, and the subsequent phase separation makes the spinel and Ti oxides crystallized, we suggest that the Ti oxide is reduced by the addition of Mg to produce spinel oxides, and the locally enriched titanium also contribute to the formation of TiN. We think that the differences in the mechanisms can be also attributed to the differences in the experimental methods. In our experiments, equiaxed grain structures were formed even though the solubility product of Ti and N was at half of the required value (sample No. 9). In the experiments performed by Fujimura et al., the metal samples were melted with the slag containing MgO, and then Al and Ti were added and held for several minutes before solidification. In our experiments, Mg, which shows an excellent ability of deoxidation, was added immediately before solidification, and the time interval between the addition of Mg and solidification was small. Hence, it is thought that the change of oxide structures promoted the crystallization of TiN and that equiaxed grain structures were formed during the interim period.
The grain refinement of the solidification macrostructure in high-purity ferritic stainless steel was experimentally investigated using Ti and Mg, and the following results were obtained.
(1) The formation of equiaxed structures was promoted when the Ti content was large. The addition of Ti contributed both to the crystallization of TiN, which acted as nucleation sites of δ-ferrite, and expansion of the constitutional undercooling region.
(2) By adding Mg, the formation of equiaxed structures was greatly promoted and the Ti content could be reduced for the formation of equiaxed structures. The addition of Mg is assumed to contribute to the creation of Al2MgO4 and TiN by the reduction of Ti oxides. The inclusions, which acted as nucleation sites, showed complex layered structures, and good lattice structure coherency was observed between Mg2TiO4//Al2MgO4 and Al2MgO4//TiN. In addition, considering that the formation of equiaxed structures was suppressed at high Al content, the importance of deoxidizing conditions in controlling the macrostructure was demonstrated.