1. Introduction
It is well known that the accumulation of copper in the recycling process of Cu-contained scrap is a significant problem, because it is difficult to remove copper from the molten iron or steel by oxidizing- or reducing- refining during the present steelmaking processes. Nevertheless, some investigations have been made and a number of suggestions have been proposed for the removal of copper in steel scrap, molten iron or steel.
Sodium sulfide or sodium sulfate fluxes have been considered to remove copper in liquid iron. Imai and Sano,1) and Wang et al.2) investigated the copper distribution between sulfide flux and carbon saturated iron melts. It was found that sodium sulfide is one of the best components for decopperization, and the copper distribution ratio could be raised up to 24. O2–Cl2 gas mixture was utilized by Matsumaru et al.3) to chloridize copper contained in iron-based scrap. In this process, copper was chloridized and evaporated as copper chlorides with high vapor pressure, and iron was oxidized to remain in the form of solid. Sasabe et al.4) proposed and experimentally examined the possibility of removal of copper from molten iron by using FeCl2. In this process, FeCl2 was injected into molten iron. The iron chloride was vaporized in molten iron and reacted with copper. The experimental results showed that 0.7 and 0.06% of copper contents were decreased to 0.5 and 0.04%, respectively, under 23 kPa in partial pressure of FeCl2 at 1723 K. Based on the finding that blowing of NH3 gas onto molten copper causes its evaporation of enormously high rate, Hidani and Takemura et al.5) have attempted the removal of copper in molten steel by NH3 gas blowing under low pressure. The mechanism of decopperization was considered that gaseous CuH was produced and easy to evaporate from molten steel. Recently, the oxidative removal of copper from carbon-saturated iron via Ag phase has been proposed by Yamaguchi and Ono et al.6,7) By this way, Ag was used just as a solvent for Cu because Ag and Fe are immiscible and Cu is more easily oxidized than Ag. Copper in molten iron could be transferred to Ag phase and removed into oxide flux (such as B2O3 flux) by the oxidation reaction of copper on the interface between Ag phase and oxide flux.
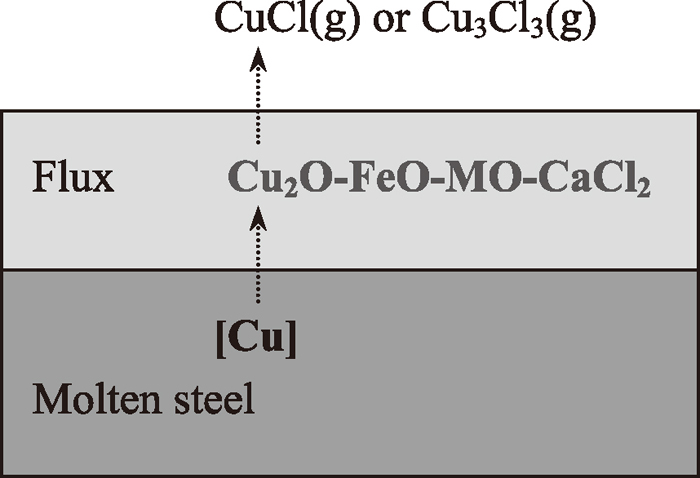
In our previous work,8) the selective chlorination of copper for Cu2O–FeO mixture by CaCl2 has been investigated. The results of thermodynamic calculation and experiments have demonstrated that copper in the Fe–Cu–O system could be selectively chlorinated by CaCl2 and removed from the reaction system. Based on the previous results, a new process for the removal of copper in molten steel will be proposed, which is shown schematically in Fig. 1. When the oxidizing flux is added onto the surface of molten steel, the copper in molten steel is partly oxidized and transferred to molten flux as Cu2O. Cu2O in molten flux is chlorinated by CaCl2 according to the principle of selective chlorination reaction. Finally, copper chlorides with high vapor pressure,9) such as CuCl or Cu3Cl3, evaporate from molten flux. So, copper in molten steel is removed by this selective chlorination and evaporation.
In this paper, FeO–SiO2–CaCl2 flux was selected to remove copper in molten steel, considering the acidic SiO2 may promote the chlorination and evaporation reaction thermodynamically. So, the chlorination and evaporation behavior of Cu2O–FeO–SiO2–CaCl2 system was firstly researched experimentally, and also experiments of decopperization for molten steel containing copper have been attempted.
2. Experimental
The experiments included two parts.
One was the chlorination and evaporation reaction of Cu2O–FeO–SiO2–CaCl2. Firstly, FeO-2 mass% Cu2O was prepared by mixing 0.48 g Cu2O and 23.52 g FeO (produced by sintering Fe3O4 and Fe powders with mole ratio 1:1 in iron crucible at 1373 K for 12 hours under N2 gas atmosphere) and heating in Al2O3 crucible at 1073 K for 8 hours under N2 gas atmosphere. Then, SiO2 and CaCl2 powders were mixed with the prepared FeO–Cu2O with mole ratio of Cu2O/CaCl2/SiO2=1:1.5:1.5 (CaCl2 is 50% excess). About 1.036 g of sample was put into an Al2O3 crucible and the crucible was inserted into a hot zone of an electric furnace (FeCrAl coil) flowed by N2 as carrier gas. At 1273 K, after reacted with 15, 30, 45, 60, 90 and 120 minutes, respectively, crucibles were taken out and weighted, and the residues in the crucible were chemically analyzed by atomic absorption spectrophotometer. Table 1 showed the details of experimental conditions and weight change of samples.
Table 1.Experimental conditions and weight change of samples.
| No. | time/min | weight/g |
|---|
| crucible | FeO–Cu2O | CaCl2 | SiO2 | crucible+residues | residues |
|---|
| 1-1 | 15 | 9.9903 | 1.0002 | 0.0233 | 0.0127 | 10.9541 | 0.9638 |
| 1-2 | 30 | 9.8095 | 1.0002 | 0.0235 | 0.0127 | 10.8169 | 1.0074 |
| 1-3 | 45 | 9.8540 | 1.0001 | 0.0235 | 0.0126 | 10.8400 | 0.9860 |
| 1-4 | 60 | 9.8744 | 1.0000 | 0.0233 | 0.0126 | 10.9105 | 1.0361 |
| 1-5 | 90 | 9.8673 | 1.0000 | 0.0236 | 0.0129 | 10.8963 | 1.0290 |
| 1-6 | 120 | 9.9115 | 1.0002 | 0.0233 | 0.0127 | 10.9276 | 1.0161 |
*all experiments were conducted at 1273 K.
Another is the decopperization for molten steel containing copper. About 100 g of solid steel was put into MgO crucible and the crucible was inserted into a MoSi2 furnace (the diameter of Al2O3 reaction tube is 80 cm). When the temperature was increased to 1873 K and ensuring steel melted, some copper was added into molten steel by a quartz tube. After fully mixed, a sample of steel was taken out by a fine quartz tube as the initial content of copper in molten steel. Then, about 15 g of the pressed block of FeO–SiO2–CaCl2 was added onto the surface of molten steel. Reacted with 10 minutes, the second sample was taken out for chemical analysis. The details of experimental conditions and the results of chemical composition were shown in Table 2.
Table 2.Experimental conditions, chemical composition, and removal ratio of copper.
| No. | weight of sample/g | copper content/wt% | removal ratio of copper/% |
|---|
| steel | flux | initial | after 10 min |
|---|
| 2-1 | 100.05 | 15.01 | 1.87 | 1.11 | 40.64 |
| 2-2 | 99.61 | 15.00 | 1.29 | 0.74 | 42.64 |
3. Results and Discussion
3.1. The Evaporation Ratios of Cu, Fe and Ca in Cu2O–FeO–SiO2–CaCl2 System
Tables 1 and 3 gave the weigh changes and chemical compositions of samples before and after reaction. From these data, the evaporation ratios of Cu, Fe and Ca were calculated by Eq. (1) and shown in Table 3.
|
R
i
eva
=
w
0,i
-
w
i
w
0,i
| (1) |
where,
R
i
eva
(mass%) is the evaporation ratio of element
i;
w0,i and
wi (g) are the amounts of element
i in the initial sample and residues, which can be determined using chemical composition and weight of samples; and
i represents Cu, Fe and Ca, respectively.
Table 3.Results of chemical composition, evaporation ratio of elements and Cu/Fe in residues.
| No. | chemical composition of residues/wt% | evaporation ratio of elements/% | Cu/Fe in residues |
|---|
| Cu | Fe | Ca | Cu | Fe | Ca |
|---|
| 1-1 | 0.49 | 66.54 | 0.74 | 71.65 | 10.58 | 8.38 | 0.0074 |
| 1-2 | 0.44 | 66.10 | 0.73 | 75.11 | 12.61 | 7.41 | 0.0066 |
| 1-3 | 0.35 | 68.51 | 0.75 | 80.63 | 11.34 | 14.60 | 0.0051 |
| 1-4 | 0.30 | 64.55 | 0.78 | 82.74 | 12.20 | 5.55 | 0.0046 |
| 1-5 | 0.23 | 65.54 | 0.71 | 86.68 | 11.46 | 14.64 | 0.0035 |
| 1-6 | 0.21 | 66.13 | 0.77 | 87.88 | 11.81 | 12.21 | 0.0032 |
*Cu/Fe ratio in the initial sample is 0.0235.
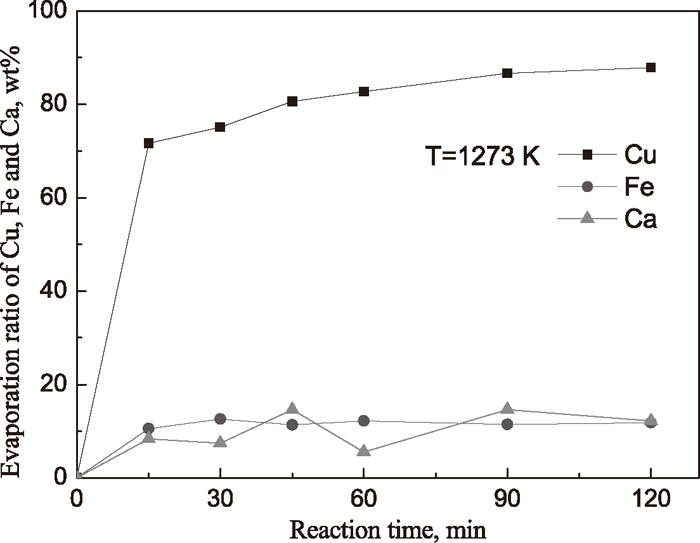
Figure 2 showed the change of evaporation ratio of Cu, Fe and Ca with time at 1273 K for Cu2O–FeO–SiO2–CaCl2 system. It was shown that the evaporation ratios of Cu increased with the reaction time. After 15 minutes, the evaporation ratio of Cu was greater than 70%, and reached up to about 88% with 2 hours. Compared with our previous results, 55.5% at 1273 K and 4 hours for Cu2O–FeO–CaCl2 system,8) the evaporation ratio of Cu was significantly enhanced, and simultaneously the evaporation ratios of Fe and Ca were both kept about 10%.
The reasons caused the promoted effect can be considered as two points. One is the additive of SiO2. Adding SiO2 as acidic oxide composition into the reaction system, thermodynamically, the following chlorination and evaporation reaction was occurred more easily.
|
(C
u
2
O)+CaC
l
2
(l)+(Si
O
2
)→CuCl(g) or C
u
3
C
l
3
+CaO⋅Si
O
2
(s)
| (2) |
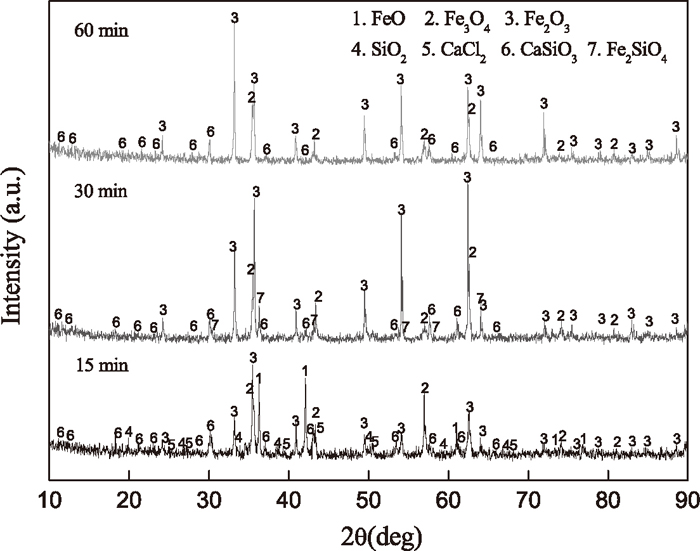
The results of XRD analysis for the residues with reaction time 15, 30 and 60 minutes, shown in
Fig. 3, indicated that CaO·SiO
2 was indeed formed during the reaction process. Another reason is that the excessive amount of chlorinating agent ensured the chlorination process reached to an enough extent.
Figure 4 showed the change of Cu/Fe ratio in residues with reaction time for Cu2O–FeO–SiO2–CaCl2 system. It was shown that Cu/Fe ratio in the reacted sample decreased from the initial 0.023 to the final 0.003 with increasing reaction time to 2 hours, which means that the copper content in molten steel decreases from about 2.3 wt% to 0.3 wt% if the reacted sample is utilized as a secondary iron source to produce steel.
3.2. The Decopperization for Molten Steel by FeO–SiO2–CaCl2 Flux
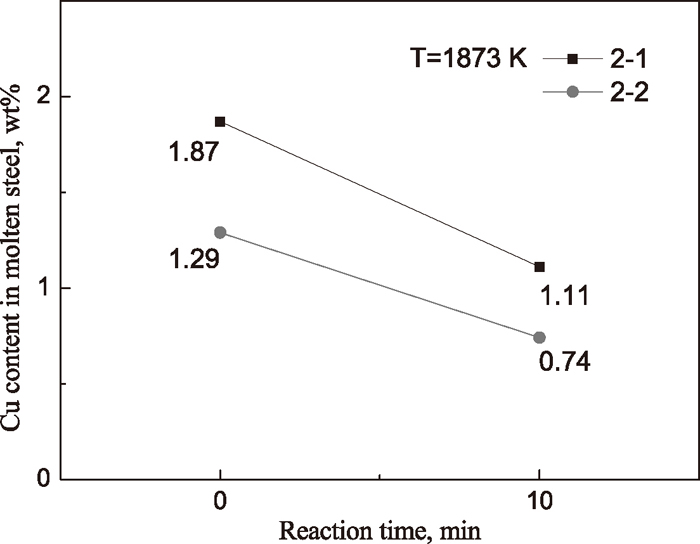
Table 2 and Fig. 5 showed two experimental results of decopperization for molten steel by FeO–SiO2–CaCl2 flux at 1873 K. For the experiment No. 2-1, the copper content in molten steel decreased from 1.87 to 1.11 wt%, and for the experiment No. 2-2, the copper content decreased from 1.29 to 0.74 wt%. The removal ratios of copper were calculated by
|
R
Cu
=
c
10
-
c
0
c
0
×100%
| (3) |
where,
c0 and
c10 are the copper contents in initial time and 10 minutes, respectively. The calculated result showed that 40.64% and 42.64% of copper in molten steel were removed with 10 minutes for two steel samples, respectively. It is fairly satisfactory, compared with the result by Sasabe
et al.,
4) which the removal ratio of copper is about 10–20% with 60 minutes.
The decopperization reaction is so intense that the flux was just added onto the molten steel, the green flame appeared shortly. According to the characteristic color reaction of copper, the green flame color indicates the copper has evaporated from the molten steel. The decopperization process has being shown in Fig. 1. On the interface between molten steel and molten flux, the dissolved copper in molten steel was partly oxidized and entered into the flux as Cu2O according to reaction (4). In molten flux, Cu2O was chlorinated by CaCl2 to CuCl(g) according to reaction (5), but FeO could not be chlorinated and remained in reaction system according to the principle of selective chlorination reaction. So, the total reaction was expressed as reaction (6).
|
2
[Cu]
in steel
+(FeO)=(C
u
2
O)+Fe(l)
| (4) |
|
(C
u
2
O)+CaC
l
2
(l)=2CuCl(g)+(CaO)
| (5) |
|
2
[Cu]
in steel
+(FeO)+CaC
l
2
(l)=2CuCl(g)+(CaO)+Fe(l)
| (6) |
The reaction (4) is thermodynamically difficult to occur in the standard state, but actually a small amount of produced Cu2O in flux can immediately react with CaCl2 and the dissolved copper in molten steel will continue to be removed.
4. Conclusions
According to the principle of selective chlorination and evaporation reaction, a new process for the removal of copper in molten steel was proposed. On the basis of researches on the chlorination and evaporation behavior of Cu2O–FeO–SiO2–CaCl2 system, FeO–SiO2–CaCl2 flux was selected to remove copper in molten steel. The conclusions are as follows:
(1) At 1273 K, for Cu2O–FeO–SiO2–CaCl2 system, the evaporation ratios of Cu increased with the reaction time. The evaporation ratio of Cu was 70% with 15 minutes and 88% with 2 hours, and simultaneously the evaporation ratios of Fe and Ca were both kept about 10%. Cu/Fe ratio in the reacted sample decreased from the initial 0.023 to the final 0.003.
(2) At 1873 K, the copper in molten steel was removed by using FeO–SiO2–CaCl2 flux. One experiment showed the copper content in molten steel decreased from 1.87 to 1.11 wt%, and another experiment showed the copper content decreased from 1.29 to 0.74 wt%. The removal ratios of copper in molten steel were 40.64% and 42.64%, respectively, with 10 minutes.
Acknowledgements
The author would like to acknowledge financial support from the National Natural Science Foundation of China (No.51104071).
References
- 1) T. Imai and N. Sano: Tetsu-to-Hagané, 74 (1988), 640.
- 2) C. Wang, T. Nagasaka, M. Hino and S. Ban-ya: ISIJ Int., 31 (1991), 1300.
- 3) K. Matsumaru, M. Susa and K. Nagata: Tetsu-to-Hagané, 82 (1996), 799.
- 4) M. Sasabe, E. Harada and S. Yamashita: Tetsu-to-Hagané, 82 (1996), 129.
- 5) T. Hidani, K. Takemura, R. O. Suzuki and K. Ono: Tetsu-to-Hagané, 82 (1996), 135.
- 6) K. Yamaguchi, H. Ono and T. Usui: Tetsu-to-Hagané, 96 (2010), 531.
- 7) K. Yamaguchi and H. Ono: ISIJ Int., 52 (2012), 18.
- 8) X. J. Hu, P. G. Jiang, Z. Yan, L. Q. Zhu, K. C. Chou, H. Matsuura and F. Tsukihashi: ISIJ Int., 53 (2013), 541.
- 9) I. Barin and O. Knacke: Thermochemical Properties of Inorganic Substances, Springer-Verlag, Berlin, (1973), 259, 260.