2013 Volume 53 Issue 7 Pages 1161-1164
2013 Volume 53 Issue 7 Pages 1161-1164
Thermodynamic information on the equilibrium between dissolved Cr and O in steel melt is the basic knowledge to control Cr content in the stainless steel production process. In the case of using alumina refractories, molten Fe–Cr alloy equilibrates locally with ‘FeO•(Cr, Al)2O3’ solid solution at the interface between the melt and refractories when Cr content in the alloy is low. Wide composition range of ‘FeO•(Cr, Al)2O3’ solid solution is known, and the activity data of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution is required to precisely estimate and control the Cr–O equilibrium relation in molten Fe–Cr when the alloy is produced in the furnace lining by Al2O3 refractories. In the present work, crucibles made of ‘FeO•(Cr, Al)2O3’ solid solution were equilibrated with molten Ag under CO–CO2 gas mixture at 1873 K to determine the activity of FeO•Cr2O3 in the solid solution.
Various elements are added into steel in order to achieve the desired physical, mechanical and chemical properties of final products. For instance, Cr is added in tool steel and stainless steel. In the refining process of Cr added steel, oxidation loss of Cr could easily occur from metal to slag due to its less-noble nature. Hence, it is very important to improve the yield of Cr. Thermodynamic information on the equilibrium between dissolved Cr and O in steel melt is the basic knowledge to control Cr content in the Cr added steel production process. Due to such background, one of the authors have reported the thermodynamic study on Fe–Cr–O system and clarified the equilibrium relation between Cr and O contents on the conditions of Cr oxides equilibria.1,2) It was found that Cr oxide phases that equilibrated with molten Fe–Cr alloy changed with Cr content. The critical Cr content in Fe–Cr alloy was approximately 7 mass% at 1873 K. The oxide in equilibrium was Cr2O3 when the Cr content was higher than this critical content and it changed to ‘FeO•Cr2O3’ when Cr content was lower than the critical value. It was found that the oxide in contact did strongly affect the equilibrium O contents against Cr contents in Fe–Cr alloy. Therefore, reaction between Fe–Cr alloy and refractories should be taken into account to control the impurity elements, because their refining reactions utilize oxidation or reduction. When Al2O3 refractories are used, Fe–Cr alloy equilibrates with either (Cr, Al)2O3 or ‘FeO•(Cr, Al)2O3’ solid solution.3) Hence, the equilibrium with either Cr2O3 based solid solution must be considered when Al2O3 refractories are used for Fe–Cr alloy refining. The equilibrium relation between Cr and O in molten Fe–Cr alloy coexisted with (Cr, Al)2O3 or ‘FeO•(Cr, Al)2O3’ solid solution was clarified at 1873 K in the previous work.3) Excellent agreement was observed between the experimental and the estimated results based on the melt-(Cr,Al)2O3 solid solution equilibrium in high Cr alloy experiments. However, discrepancy was observed in low Cr alloy experiments. The reason for the disagreement was considered to be due to difference of the estimated FeO•Cr2O3 activity in ‘FeO•(Cr, Al)2O3’ solid solution. Therefore, the activity of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution was measured to precisely express the equilibrium relation of Cr and O contents in molten Fe–Cr alloy in contact with Al2O3 based refractories in the present work.
The activity of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution was measured by equilibrating ‘FeO•(Cr, Al)2O3’ crucible with molten Ag under purified CO–CO2 gas mixture in an electric resistance furnace. Crucibles were prepared as follows. ‘FeO’ was made by mixing equivalent molar electrolytic Fe powder with reagent grade Fe2O3 powder and heating the mixture in a Fe crucible at 1473 K under purified Ar atmosphere for 6 h. Magnetic separation was used to remove metallic Fe and Fe3O4 from the homemade ‘FeO’ after crushing. The formation of ‘FeO’ was confirmed by XRD. Powders of ‘FeO•Cr2O3’ and ‘FeO•Al2O3’ were made as follows. Reagent grade Cr2O3 or Al2O3 powder was mixed with ‘FeO’ and was pressed into tablet shape. These tablets were held in a Pt crucible at 1573 K under purified Ar atmosphere for 6 h. The tablets were quenched and crushed into powders. The powders were pressed and heated again under the same condition. The formation of ‘FeO•Cr2O3’ and ‘FeO•Al2O3’ were confirmed by XRD. Mixed molar ratios of ‘FeO’ and Cr2O3 were 0.5:0.5, 0.6:0.4 or 0.7:0:3. Mixed molar ratio of ‘FeO’ and Al2O3 was 0.5:0:5. These ratios were chosen based on the psudo-binary phase diagrams of FeO–Cr2O3 and FeO–Al2O3.4,5) Solid solution range of ‘FeO•Cr2O3’ is wide from approximately 70 mass% Cr2O3 in FeO–Cr2O3 binary to almost stoichiometric composition of 43 mass% Cr2O3 coexisting with pure Cr2O3 at 1873 K.4) In contrast, solid solution range of ‘FeO•Al2O3’ is very narrow at 1873 K.5) Powders of ‘FeO•Cr2O3’ and ‘FeO•Al2O3’ were mixed and pressed into tablet shape. Those tablets were held in a Pt crucible at 1723 K under purified Ar atmosphere for 6 h. The tablets were quenched and crushed into powder. The powders were pressed and heated again under the same condition. The formation of ‘FeO•(Cr, Al)2O3’ were confirmed by XRD. Mixed molar ratios of ‘FeO•Cr2O3’ and ‘FeO•Al2O3’ were 1.0:0, 0.8:0.2, 0.6:0.4, 0.4:0.6 or 0.2:0.8. Synthesized ‘FeO•(Cr, Al)2O3’ powders were pressed into crucible shape (O.D. 16 mm, I.D. 12 mm, H 10 mm) by rubber pressing machine and were sintered in a Pt crucible at 1723 K under purified Ar atmosphere for 6 h.
Experimental details have been described elsewhere.6) Synthesized crucible with approximately 4 g of Ag was placed on Mo sheet set inside an Al2O3 crucible. This sample was heated at 1873K in a vertical electric resistance furnace with LaCrO4 heating elements. The reaction tube was of Al2O3, 60 mm in ID and 1000 mm length. Gas mixture of CO-5.04 vol%CO2 was blown on molten Ag placed inside a ‘FeO•(Cr, Al)2O3’ crucible. After 4 h, predetermined time required for equilibrium, the sample was quickly pulled out from the furnace and was quenched by impinging He gas. The Fe and Cr contents in Ag were determined by an induction coupled plasma spectroscopy (ICP). Chemical composition of crucible interface equilibrated locally with molten Ag was determined by SEM-EDS.
The activity of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution was determined as follows. Reaction involving Fe, Cr dissolved in molten Ag, FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution, CO and CO2 and its Gibbs energy change, ΔG°, can be expressed as Eqs. (1) and (2) using T as absolute temperature. The standard state of FeO•Cr2O3 is that saturated with Cr2O3.2,6)
| (1) |
The equilibrium constant, K, can be expressed as follows.
| (3) |
| (4) |
| 2) (5) |
| 2) (6) |
| Heat No | mass ppm Fe in Ag | mass ppm Cr in Ag | aFeO•Cr2O3 | X FeO in ‘FeO•(Cr, Al)2O3’ | XCr2O3 in ‘FeO•(Cr, Al)2O3’ | XAl2O3 in ‘FeO•(Cr, Al)2O3’ |
|---|---|---|---|---|---|---|
| 1 | 1810 | 0.79 | 0.0004 | 0.456 | 0.117 | 0.426 |
| 2 | 391 | 4.25 | 0.0026 | 0.451 | 0.180 | 0.369 |
| 3 | 1470 | 11.3 | 0.0676 | 0.482 | 0.297 | 0.220 |
| 4 | 1090 | 14.0 | 0.0779 | 0.485 | 0.397 | 0.118 |
| 5 | 1070 | 41.3 | 0.660 | 0.488 | 0.512 | 0 |
| 6 | 2110 | 0.64 | 0.0003 | 0.490 | 0.076 | 0.434 |
| 7 | 2280 | 2.49 | 0.0051 | 0.485 | 0.160 | 0.355 |
| 8 | 2320 | 6.08 | 0.0310 | 0.487 | 0.245 | 0.267 |
| 9 | 2200 | 6.32 | 0.0318 | 0.486 | 0.391 | 0.123 |
| 10 | 2490 | 12.8 | 0.147 | 0.523 | 0.477 | 0 |
| 11 | 2230 | 3.74 | 0.0113 | 0.476 | 0.067 | 0.457 |
| 12 | 1770 | 4.14 | 0.0110 | 0.482 | 0.133 | 0.385 |
| 13 | 1950 | 1.06 | 0.0008 | 0.551 | 0.187 | 0.263 |
| 14 | 2540 | 7.85 | 0.0565 | 0.535 | 0.321 | 0.145 |
| 15 | 2640 | 4.50 | 0.0194 | 0.560 | 0.440 | 0 |
The activities and iso-activity curves of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution are shown in Fig. 1. The composition of ‘FeO•(Cr, Al)2O3’ coexisting with (Cr, Al)2O3 was taken from the previous experimental results.3) FeO solubility limit of ‘FeO•(Cr, Al)2O3’ at 1923 K reported by Rosenbach and Schmitz5) was taken into account for estimating ‘FeO•(Cr, Al)2O3’ range. It is clearly observed that the activity of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution decreased rapidly with decrease of FeO•Cr2O3 content in ‘FeO•(Cr, Al)2O3’. Also, the activity of FeO•Cr2O3 decreases with increase of FeO content in ‘FeO•(Cr, Al)2O3’. It is clear that the composition change of ‘FeO•(Cr, Al)2O3’ solid solution does strongly affect the activity of FeO•Cr2O3.
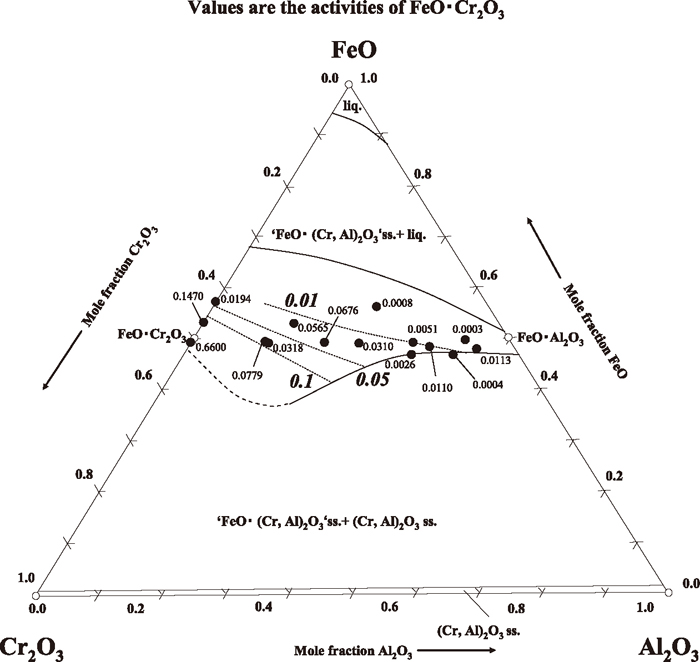
Iso-activity curves of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution at 1873 K.
As explained in our previous study,3) the equilibrium relation between Cr and O in molten Fe–Cr alloy coexisted with ‘FeO•(Cr, Al)2O3’ solid solution at 1873 K couldn’t be well expressed due to unknown FeO•Cr2O3 activity in ‘FeO•(Cr, Al)2O3’ solid solution. From the iso-activity curves of FeO•Cr2O3 in Fig. 1, the FeO•Cr2O3 activities in ‘FeO•(Cr, Al)2O3’ solid solution coexisting with molten Fe–Cr alloy and the corresponding equilibrium O contents at given Cr contents in molten Fe–Cr alloy in the previous experiment3) were estimated as shown in Table 2. The equilibrium O contents were calculated by use of Eqs. (10) and (11) of the previous work.3) As the results, the equilibrium relation between Cr and O contents in molten Fe–Cr alloy melting in a refining furnace lined by Al2O3 refractories at 1873 K is shown in Fig. 2 based on the Table 2 and the previous result3) on the Cr–O equilibrium under (Cr, Al)2O3 saturation. Also, results using Cr2O3 crucible,1,2,8) FeCr2O4 crucible8) and Al2O3 crucible9,10,11) are shown in Fig. 2. The equilibrium relation between Cr and O in molten Fe–Cr alloy coexisted with (Cr, Al)2O3 or ‘FeO•(Cr, Al)2O3’ solid solution was estimated under the condition that local equilibrium between metal and crucible at the interface was attained, initial O content was high and Al content was negligible. The equilibrium O contents at given Cr contents in molten Fe–Cr alloy equilibrated with Al2O3 crucible were obviously lower than those equilibrated with Cr2O3 or FeCr2O4 crucible. In the previous work,3) discrepancy was observed between the experimental and the estimated results based on the metal-‘FeO•(Cr, Al)2O3’ solid solution equilibrium in low Cr alloy experiments shown as dashed curve in Fig. 2, due to the inaccuracy of the estimated FeO•Cr2O3 activity. However, it was found that the equilibrium relation between Cr and O in molten Fe–Cr alloy coexisted with ‘FeO•(Cr, Al)2O3’ solid solution 1873K can be expressed accurately with use of the activity data of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution determined in the present work.
| Heat No | [mass%Cr]3) | aFeO•Cr2O3 | [mass%O] |
|---|---|---|---|
| 1 | 0.12 | 0.0002 | 0.021 |
| 2 | 0.19 | 0.008 | 0.024 |
| 3 | 0.56 | 0.020 | 0.032 |
| 4 | 0.85 | 0.050 | 0.033 |
| 5 | 1.02 | 0.070 | 0.033 |
| 6 | 1.72 | 0.120 | 0.032 |
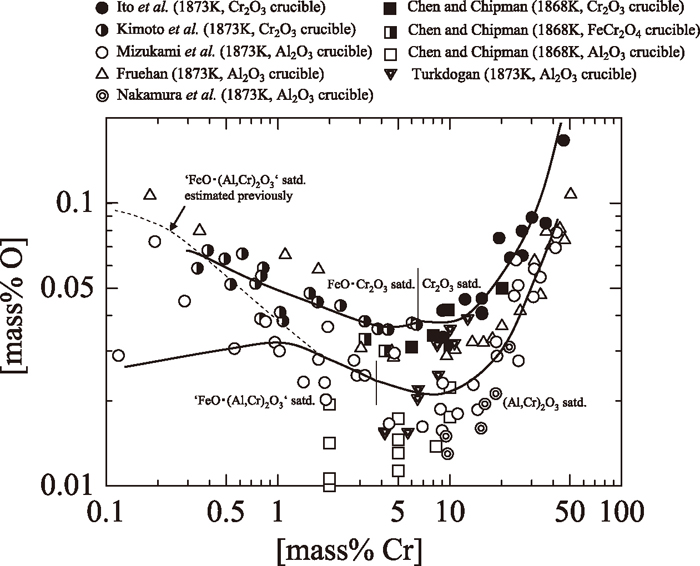
Equilibrium relation between Cr and O contents in molten Fe–Cr alloy melting in a refining furnace lined by Al2O3 refractories at 1873 K.
Crucibles made of ‘FeO•(Cr, Al)2O3’ solid solution were equilibrated with Ag under CO–CO2 gas mixture at 1873 K. The activities of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution having various composition were determined. From the result, iso-activity curves of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ were obtained. It was found that the activity of FeO•Cr2O3 decreases rapidly with decrease of FeO•Cr2O3 content and with increase of FeO content in ‘FeO•(Cr, Al)2O3’. It was made clear that the composition change of ‘FeO•(Cr, Al)2O3’ solid solution did strongly affect the activity of FeO•Cr2O3. It was found that the equilibrium relation between Cr and O in molten Fe–Cr alloy contacted with Al2O3 refractories at 1873 K could be expressed accurately with use of the activity data of FeO•Cr2O3 in ‘FeO•(Cr, Al)2O3’ solid solution and the activity data of Cr2O3 in (Cr, Al)2O3 solid solution.