2013 Volume 53 Issue 7 Pages 1253-1259
2013 Volume 53 Issue 7 Pages 1253-1259
Recently, advanced high strength steels for automotive applications were designed to achieve a carbide-free bainitic microstructure after conventional thermo-mechanical processing and a continuous annealing treatment. The microstructure obtained consists of ferrite laths interwoven with thin films of untransformed retained austenite. The sufficiently tough matrix and the control of the heterogeneity in the microstructure allowed an optimum combination of strength, ductility, and formability to be achieved. However, this work probed that even using a devoted theoretical alloying, carbide-free bainitic steels are hardly compatible with a conventional hot dip galvanizing annealing process, without any consideration about Zn coatability and adherence. The hardenability of the designed alloys was insufficient in some cases and the amount of austenite retained in the microstructure was too low or/and mechanically too unstable for high ductility. As a consequence, the obtained mechanical properties were comparable to those in high-Si dual phase steels without a beneficial transformation induced plasticity effect.
Recently, there has been increased interest in the development of the third generation of advanced high strength steels (AHSS), i.e., steels with strength-ductility combinations significantly better than exhibited by the first generation AHSS i.e. steels that possess primarily ferrite-based microstructures such as dual phase (DP), transformation-induced plasticity (TRIP),1) complex-phase (CP), and martensitic (MART) steels; but at a cost significantly less than required for second generation AHSS, i.e., austenitic steels with high manganese contents, which include steels that are closely related to austenitic stainless steels, such as twinning-induced plasticity (TWIP) steels.2) The third generation of AHSS will include materials with microstructures consisting of a high strength phase (e.g., ultra-fine grained ferrite, martensite, or bainite) and a significant amount of ductile austenite that improves the work-hardening of the complex composite structure by TRIP effect.
Approaches to the development of the third generation of bainitic AHSS have been performed.3,4,5,6,7,8) Design methodologies based on diffusionless bainite transformation theory were applied to develop steels with a carbide-free bainitic (CFB) microstructure consisting of a mixture of bainitic ferrite, retained austenite, and some martensite.9,10) Using thermodynamics and kinetics models, CFB steels with a 0.2 and 0.3 wt.% carbon content were designed and manufactured following a conventional hot rolling practice.3,4,5,6) The designed steels present significant combinations of strength and ductility, with tensile strengths ranging from 1300 to 1800 MPa and total elongations of over 14%.
In terms of in-use properties, the CFB steel is expected to achieve extremely good stretch-flangeability due to its uniform fine lath structure.11) On the other hand, the heterogeneities of hardness due to the presence of martensite in this advanced bainitic microstructure will allow this steel to reach a good, deep drawability.12) Recently, annealed cold rolled bainitic steels designed for uncoated (i.e. bare) or electrogalvanized (EG) products and manufactured by a continuous annealing line (CAL) achieved outstanding mechanical performances compared to present-day very high strength offers.7,8) They reached far higher uniform elongation, better stretching ability and formability than that in DP980 and Martensitic 1400 steels considering the same range of ultimate tensile strengths. On the other hand, thanks to the bainitic/tempered martensitic matrix, the designed steels showed very high hole expansion ratio compared to fully martensitic steels and DP steels. The designed steels presented thus simultaneously a sufficient stretchability for cold-stamping operations and very high resistance to damage processes (fracture strain, cut-edge sensitivity, bending-ability).
In this work, the possibility to achieve a CFB microstructure after a hot dip galvanizing (HDG) production line for galvanized or galvannealed products, was investigated. The thermal profile of this type of annealing line is certainly not the most suitable to produce CFB microstructures. The limitations come from the short holding to achieve the bainitic transformation and the fixed temperature of the zinc baths (about 460°C). The flexibility of this class of annealing line is thus less advantageous than CAL. Nevertheless, this option is worth being investigated for economic (cost of the final coated product compared to EG material) and technical reasons (several TRIP steels are already manufactured with HDG industrial lines).
A first set of steels (CAL1 and CAL2), specifically designed to achieve a CFB microstructure after a CAL process in a former work,8) were used. Ingots were cast using a vacuum induction melting furnace. Their actual composition is listed in Table 1. The ingots were hot rolled to a thickness of 3 mm following the thermomechanical control process of commercial SiMn TRIP steels described elsewhere.8) A high coiling temperature of 550°C was selected to trigger the ferrite-pearlite transformation region, and thus, to achieve soft hot rolled strips and to enable the cold-rolling without limiting the accessible gauge thickness for the final product. The strips were subsequently cold-rolled to a total reduction rate of 50% in multiple passes, without any cracks.
| Alloys | C | Mn | Si | Cr | P | S |
|---|---|---|---|---|---|---|
| First set of alloys:8) | ||||||
| CAL1 | 0.15 | 2.01 | 1.45 | 0.62 | 0.016 | — |
| CAL2 | 0.18 | 2.00 | 1.46 | 0.62 | 0.016 | — |
| Second set of alloys: | ||||||
| HDG1 | 0.18 | 1.02 | 1.60 | 0.91 | 0.016 | — |
| HDG2 | 0.17 | 1.47 | 1.49 | 0.74 | 0.012 | 0.007 |
A scheme of the temperature-time profile used during the HDG process for commercial manufacturing of galvanized or galvannealed products, is illustrated in Fig. 1. The simulations were performed using an Advanced Energy Technology (AET) furnace. The only process parameter that could be modified is the annealing temperature (AT). Three high annealing temperatures (830, 860 and 880°C), but inside the conventional feasibility range, have been tested for the HDG simulations. The line speed, which could not be extensively changed, has been maintained constant in this study.

Scheme of heat treatment used for simulation of HDG process including approximate cooling rate and overaging duration. AT is annealing temperature.
Before heat treatment simulation, Ac3 transformation temperature were experimentally determined by means of an Adamel Lhomargy DT1000 high-resolution dilatometer13) to be 895°C in CAL1 steel and 880°C in CAL2 steel. Dilatometric data showed that the selected annealing temperatures do not imply complete austenitization for both alloys, except for 880°C in CAL2 steel. In this sense, certain amount of intercritical ferrite is expected to be present after HGD process in both steels.
During overaging or holding stage (Fig. 1) at the molten zinc bath temperature (~460°C for 100 s – low line speed), the austenite decomposes to bainite for both steels (being MS temperature 420°C in CAL1 steel and 384°C in CAL2 steel). Kinetics results reported elsewhere8) showed that the time required to complete bainitic transformation at 450°C was 12.8 min. in CAL1 steel and 17 min. in CAL2 steel. These data anticipated insufficient bainite transformation kinetics of the first set of annealed cold rolled CFB steels to be able to produce a fully CFB microstructure by means of the HDG process.
Nevertheless, the HDG simulations were performed in both alloys and two tensile samples per heat-treated plate were machined (ISO 12.5×50 standard samples). The tensile properties are summarized in Table 2 and compared with those achieved for both steels after CAL process. All the samples of this first set of alloys after HDG process present very high UTS (1100–1400 MPa) but their uniform and total elongations are rather disappointing compared to conventional high strength steels. Their work-hardening ratio (YS/UTS~0.5) is also comparable to that in DP steels. The tensile curves show the continuous yielding and strong strain hardening typical for DP steels, as illustrated in Fig. 2(a). Finally, their mechanical performance is rather insensitive to the annealing temperature.
| Alloys | Approximate Composition | AT, °C | BHT, °C | YS, MPa | UTS, MPa | YS/UTS | UEl, % | TEl, % | UTS×TEl, MPa% |
|---|---|---|---|---|---|---|---|---|---|
| First set of alloys after CAL:8) | |||||||||
| CAL1 | 0.15C2Mn1.5Si0.6Cr | 890 | 400 | 853 | 1146 | 0.74 | 7.9 | 15.8 | 18107 |
| CAL2 | 0.2C2Mn1.5Si0.6Cr | 890 | 400 | 822 | 1232 | 0.67 | 8.5 | 15.5 | 19096 |
| 890 | 350 | 1077 | 1319 | 0.82 | 4.5 | 11.6 | 15300 | ||
| 890 | 300 | 1106 | 1394 | 0.79 | 4.8 | 11.9 | 16589 | ||
| First set of alloys after HDG: | |||||||||
| CAL1 | 0.15C2Mn1.5Si0.6Cr | 830 | 460 | 451 | 1148 | 0.48 | 8.7 | 11.4 | 13030 |
| 860 | 460 | 498 | 1132 | 0.50 | 8.0 | 10.7 | 12050 | ||
| 880 | 460 | 564 | 1248 | 0.57 | 6.8 | 10.0 | 12480 | ||
| CAL2 | 0.2C2Mn1.5Si0.6Cr | 830 | 460 | 652 | 1335 | 0.49 | 7.5 | 9.1 | 12149 |
| 860 | 460 | 782 | 1404 | 0.56 | 5.9 | 8.4 | 11789 | ||
| 880 | 460 | 792 | 1373 | 0.58 | 5.9 | 8.3 | 11323 | ||
| Second set of alloys after HDG: | |||||||||
| HDG1 | 0.2C1Mn1.5Si1Cr | 890 | 460 | 528 | 1053 | 0.50 | 10.0 | 13.1 | 13794 |
| HDG2 | 0.2C1.5Mn1.5Si0.75Cr | 890 | 460 | 602 | 1093 | 0.55 | 8.0 | 11.0 | 12023 |
AT is annealing temperatures, BHT is bainite holding temperature (i.e. overaging temperature), YS is yield strength; UTS is ultimate tensile strength; and UEl and TEl is uniform and total elongation, respectively.

(a) Tensile curves of CAL1 alloy after HDG at different annealing temperatures. The area enclosed by the circle shows continuous yielding and initial strong strain hardening typical for DP steels (b) Corresponding evolution of instantaneous strain hardening coefficient as a function of true strain. The straight line represents the onset of necking or Considère’s criterion.
The optical micrographs after Nital etching of HDG samples are presented in Fig. 3. All the samples exhibit a mixed microstructure of coarse bainite, martensite and some traces of equiaxed ferrite. Only alloys annealed at 830°C show the presence of significant amount of intercritical ferrite (25% in CAL1 steel and 15% in CAL2 steel). At this scale, it is not possible to identify the presence or not of retained austenite, but the evolution of instantaneous strain hardening coefficient as a function of true strain showed no significant TRIP effect (Fig. 2(b)).

Optical micrographs of HDG samples at different annealing temperatures (AT): (a) CAL1 steel AT=830°C; (b) CAL1 steel AT=860°C; (c) CAL1 steel AT=880°C; (d) CAL2 steel AT=830°C; (e) CAL2 steel AT=860°C; (f) CAL2 steel AT=880°C. Nital etching. F is equiaxed ferrite, B is bainite and M is martensite.
Present results are consistent with preliminary thermodynamics calculations reported elsewhere14) showing that the bainitic transformation in CAL1 and CAL2 steels at 460°C is far from being complete. Since the maximum volume fraction of bainite that can be formed is limited (to ~50% for CAL1 steel and ~70% for CAL2 steel), the carbon enriched in austenite will be insufficient for retaining a significant fraction of austenite after cooling down to room temperature. The lack of TRIP effect is thus related to the too low fraction of bainite obtained at 460°C, which disables the necessary carbon enrichment for stabilizing retained austenite at room temperature.
When compare the tensile performances of the CAL1 and CAL2 alloys after HDG process with those obtained after CAL process (see Table 2), it appears that the UTS/UEl performances of HDG alloys are similar to those of CAL alloys. Nevertheless, HDG designed steels show very low YS. This is explained by the presence of ferrite and bainite formed at higher temperature (coarse bainite).
Therefore, it was demonstrated that it is almost impossible to reach a fully CFB microstructure following a classical HDG industrial-like profile with the first set of alloys. The obtained mechanical properties are disappointing since they are very similar to those obtained with DP microstructures, without any TRIP effect and low YS/UTS ratio. The limiting parameter is kinetics and thermodynamic for the studied alloys. As a consequence, an adjustment on the alloy design of annealed cold rolled steels compatible with a HDG cycle is required.
An alloy design procedure3) based on thermodynamics and kinetics theory was applied to shift bainitic region in the TTT diagram to higher temperatures and to shorter transformation time in CAL2 reference steel (0.2C–1.5Si–2Mn–0.6Cr wt%).
3.1. Outline of Phase Transformation Models in SteelsThe bainite transformation progresses by the diffusionless growth of tiny platelets known as ‘sub-units’.15) The excess carbon in these platelets partitions into the residual austenite soon after the growth event. Diffusionless growth of this kind can only occur if the carbon concentration of the residual austenite is below that given by the To curve. The To curve is the locus of all points, on a temperature versus carbon concentration plot, where austenite and ferrite of the same chemical composition have the same free energy.16) It follows that the maximum amount of bainite that can be obtained at any temperature is limited by the fact that the carbon content of the residual austenite must not exceed the To curve of the phase diagram. The design procedure avoids this difficulty in two ways: by adjusting the To curve to greater carbon concentrations with the use of substitutional solutes such as Mn and Cr and by controlling the mean carbon concentration.9,10)
Bainite is formed below the To temperature when ΔGγ→α < –GSB and ΔGm < GN, where GSB ≅ 400 J mol–1 is the stored energy of bainite16) and ΔGγ→α is the free energy change accompanying the transformation of austenite without any change in chemical composition. The first condition therefore describes the limit to bainite growth. The second condition refers to nucleation; thus, ΔGm is the maximum molar Gibbs free energy change accompanying the nucleation of bainite. GN is a universal nucleation function based on a dislocation mechanism of the kind associated with martensite.17) The temperature dependence of GN is independent of chemical composition; together with the growth condition, the function allows the calculation of the bainite start temperature, BS, from a knowledge of thermodynamics alone.
Apart from controlling the To curve and BS temperature, substitutional solutes also affect hardenability, which is an important design parameter to avoid transformations such as proeutectoid ferrite and pearlite. For this purpose, thermodynamic and kinetics models developed to allow the estimation of isothermal and continuous transformation diagrams, from the knowledge of the chemical composition of the steel concerned, were used in the alloy design process.18,19,20,21,22) There are other output parameters, such as the martensite and Widmanstätten start temperatures. An extensive description of all the models used on the design procedure was reported elsewhere.3)
3.2. Theoretical Calculations and Alloy DesignThe Cr content was determined in the alloy systems 0.2C–1.5Si–(1.0/1.5)Mn–XCr to shift the bainitic nose to a temperature of ~460°C (molten zinc bath temperature). In addition, calculations on the driving force for bainite transformation of the new designed alloys were performed in order to ensure that the readjustment of chemical composition did not result in an increase on the bainite reaction time.
Results on the influence of Cr additions on the temperature at the bainitic nose in the TTT diagram (see Figs. 4(a) and 4(b)) of Fe–0.2C–1.5Si–1.0/1.5Mn–XCr alloy systems, suggest that Cr additions within the range (0.75–2.25%) for Fe–0.2C–1.5Si–1.0Mn–XCr and (0.25–1.25%) for Fe–0.2C–1.5Si–1.5Mn–XCr are recommended to reach the shortest bainitic reaction time at temperature close to 460°C. Corresponding driving force calculations (see Figs. 4(c) and 4(d)) confirm that these Cr additions are acceptable in terms of bainite reaction kinetics compared to that in the reference CAL2 steel. However, calculations on the ferrite/pearlite transformation region in the TTT diagram suggested that Cr additions lower than 1.0% in Fe–0.2C–1.5Si–1.0Mn–XCr alloy system might result on the deterioration of hardenability in the steel.
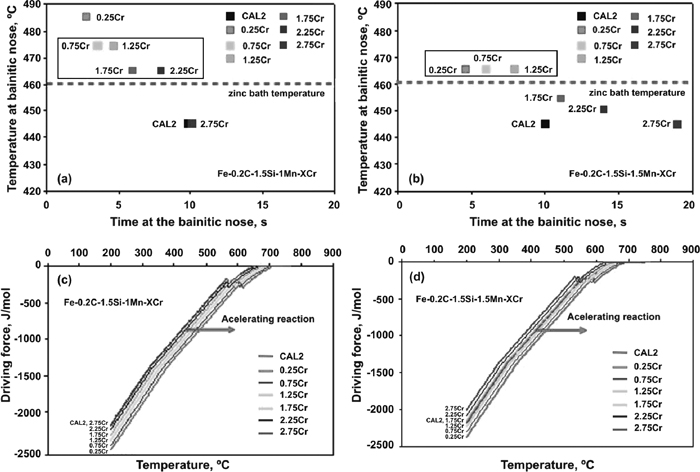
Influence of Cr additions on (a) and (b) transformation time at the bainitic nose of the TTT diagram and (c) and (d) driving force for bainite formation in Fe–0.2C–1.5Si–1.0/1.5Mn–XCr alloy system.
Finally, the chemical compositions Fe–0.2C–1.5Si–1.0Mn–1.0Cr and Fe–0.2C–1.5Si–1.5Mn–0.75Cr were selected for HDG simulation experiments without any consideration on coatability. Two new casts, HDG1 and HDG2 (see chemical composition in Table 1) were manufactured. Both ingots were hot-rolled to achieve soft hot rolled material. Afterwards, the strips were cold-rolled down to a total reduction rate of 50% (multiple passes), without any cracks.
3.3. Preliminary Dilatometer StudyThis study was firstly performed to assess the bainitic transformation kinetics of HDG1 and HDG2 steels. The thermal cycle consists mainly of a soaking at 950°C during 3 min. to guaranty a fully austenitic state prior bainitic transformation followed by a rapid cooling down to the transformation temperature, 460°C i.e. approximate temperature of Zn baths in a HDG cycle. The bainitic holding plateau is reached after ~200 s. The dilatometric results revealed that HDG1 and HDG2 alloys presents faster bainitic transformation at 460°C (~70 s. are needed to reach the stasis in both alloys) than that in CAL1 and CAL2 alloys.
The transformation product at 460°C in HDG1 and HDG2 steels was formed by CFB (darker regions in example of Fig. 5) and small high carbon martensite-austenite (MA) islands/films of retained austenite (white grains in example of Fig. 5). Therefore, preliminary dilatometer study suggested that HDG1 and HDG2 alloys were able to reach a significant amount of bainite during a short isothermal holding at 460°C and therefore a suitable carbon enrichment of residual austenite is expected.
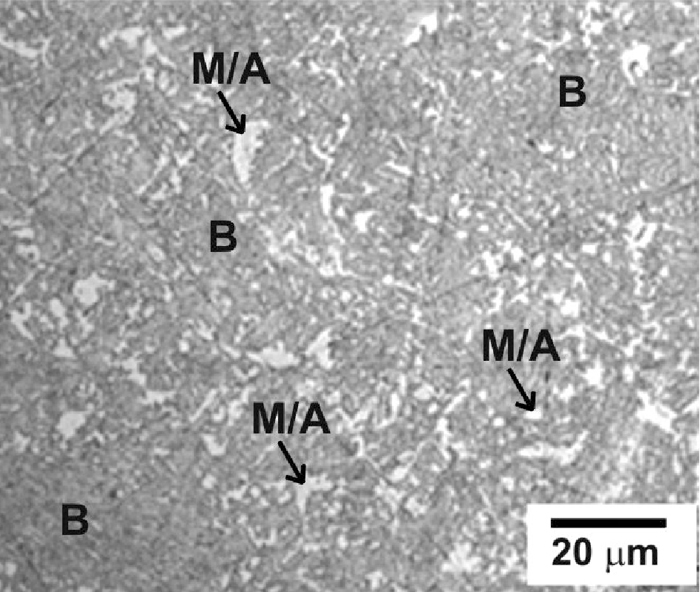
Optical micrograph of dilatometric sample isothermally transformed at 460°C in HDG1 steel. Bisulphate etching. B is bainite and M/A is martensite-austenite constituent.
Laboratory annealing simulations following an industrial-like HDG annealing cycle were performed using the same AET furnace. The thermal cycle is schematized in Fig. 1, but this time only a soaking temperature of 890°C, a high bound in conventional industrial conditions, was tested. Optical and SEM micrographs of the resulting microstructures are shown in Fig. 6. Two tensile samples per heat-treated plate were machined (ISO 12.5×50 standard samples). All the results show a good reproducibility. The corresponding results are listed in Table 2.
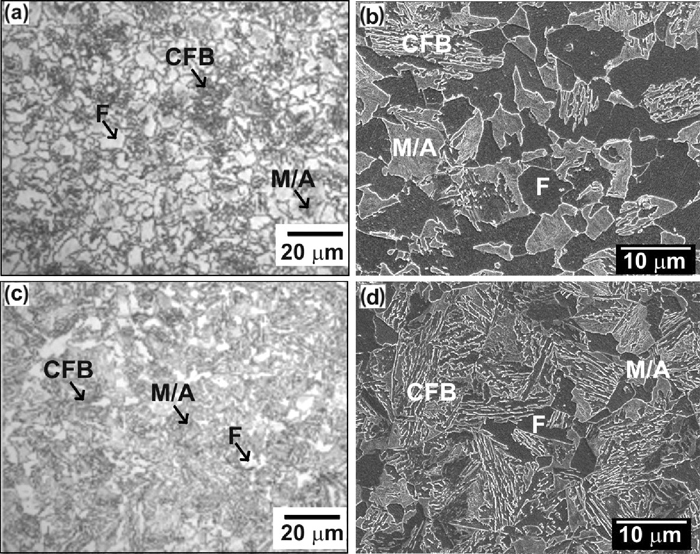
Optical and SEM micrographs of annealed cold rolled steels after HDG process: (a) and (b) HDG1 steel; and (c) and (d) HDG2 steel. Annealing temperature of 890°C. Nital etching. F is polygonal ferrite, CFB is carbide-free bainite and M/A is martensite-austenite constituent.
Quantitative data on their microstructure (See Table 3) revealed that HDG1 and HDG2 steels after HDG annealing contain significant fraction of polygonal ferrite (10%–40%) localized at the prior austenite grain boundaries. The presence of ferrite explains the low yield strengths achieved in both steels. Apart of polygonal ferrite, the microstructure in both cases is formed by laths of ferrite, films of retained austenite and martensite. HDG2 alloy contains however higher fraction of bainite than HDG1, thus closer to the targeted microstructure.
| Alloy | Vαequiaxed | Vαbainitic | VM | Vγ | xγ [wt-%] | Hardness HV5 |
|---|---|---|---|---|---|---|
| HDG1 | 0.41 ± 0.03 | 0.15 ± 0.12 | 0.42 ± 0.09 | 0.02 ± 0.03 | — | 321 ± 10 |
| HDG2 | 0.17 ± 0.02 | 0.45 ± 0.08 | 0.26 ± 0.06 | 0.12 ± 0.03 | 0.85 ± 0.05 | 440 ± 7 |
Vαequiaxed: volume fraction of equiaxed ferrite; Vαbainitic: volume fraction of bainitic ferrite; VM: volume fraction of martensite; Vγ: volume fraction of retained austenite; xγ: austenite carbon content.
The tensile performances of the studied alloys after HDG process were compared in Fig. 7 to those obtained after CAL process and to those of commercial high strength steels.23) In general, the tensile performances of HDG1 and HDG2 steels are still below expectations. They reached UEl lower than that in DP 780 grades and limited improved UTS.
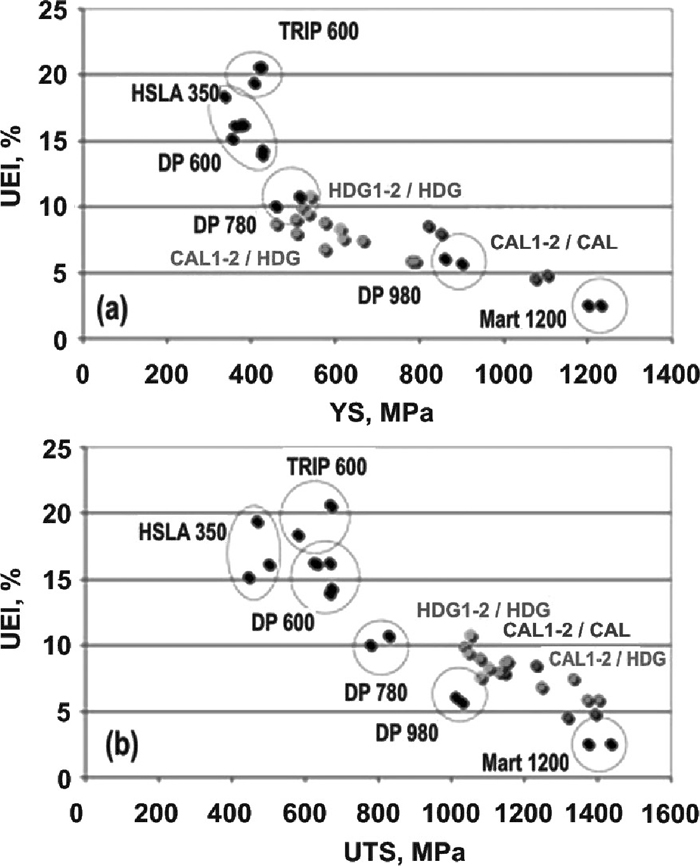
Comparison between the tensile performance of the designed alloys after CAL8) and HDG processes and other steels used for cold-stamping or cold-forming products:23) (a) uniformed elongation (UEl) as a function of yield strength (YS); and (b) uniformed elongation (UEl) as a function of ultimate tensile strength (UTS).
The main conclusions concerning the design of CFB steels compatible with an HDG annealing are the following:
(1) Even with a devoted theoretical alloy design procedure, it seems difficult to identify a lean chemical concept suitable to fulfil the following conditions:
a. A low Ac3 temperature to achieve a fully austenitic soaking.
b. Enough hardenability to avoid proeutectoid ferrite formation upon cooling.
c. To reach a sufficient carbide-free bainitic transformation at 460°C using low Mn content.
d. To increase the bainitic transformation kinetic at 460°C to be compatible with short overageing duration.
(2) As a consequence, the tensile properties of all the studied alloys are disappointing and comparable to those of high-Si DP steels.
(3) Independently of the substrate metallurgical choice, the question of the coat-ability will be raised, especially in high-Mn and high-Si chemical concepts and will require dedicated study depending on annealing furnace technology.
As concluding words, conventional HDG annealing cannot be recommended to produce fully CFB steels (i.e. without the presence of equiaxed ferrite in the microstructure), unless some improvements on soaking and cooling capabilities were explored. In this sense, Li et al.24) confirmed that a HDG annealing with a low end-cooling strategy, consisting of an overageing at temperatures lower than 460°C and followed by an induction heating, could be an option to achieve CFB microstructures with properties comparables to those obtained with CAL capabilities.
The authors gratefully acknowledge the support of the Research Fund for Coal and Steel (contract RFSR-CT-2008-00021) and the Spanish Ministry of Science and Innovation (contracts MAT2010-15330 and IPT-2012-0320-420000) for funding this research.