2013 Volume 53 Issue 7 Pages 1268-1274
2013 Volume 53 Issue 7 Pages 1268-1274
Al effects on strain aging and resistance against hydrogen embrittlement were examined in Fe–18Mn–0.6C-based twinning-induced plasticity steels deformed at different strain rates. These steels showed a hydrogen-induced fracture when they were pre-deformed at a strain rate of 1.7×10–6 s–1. This fracture was suppressed by increasing the strain rate and Al content. The two important factors for improving the resistance to hydrogen embrittlement from the viewpoint of material strengthening by strain aging were found to be (1) the suppression of dynamic strain aging by increasing the strain rate and Al content, and (2) the suppression of static strain aging under loading by the Al addition.
Fe–Mn–C-based twinning-induced plasticity (TWIP) steels show high elongation and high strength.1,2,3) However, hydrogen embrittlement has also been reported in the deformed TWIP steels.3,4,5,6,7,8,9,10,11,12,13) Hydrogen embrittlement occurs when hydrogen is introduced into the materials by corrosion, and it becomes a critical problem when the TWIP steels with significant residual stress are used for automobile parts.3) Hydrogen embrittlement is generally reported to occur when the material strength is sufficiently high. For example, the hydrogen-induced intergranular fracture was observed in a Fe–18Mn–0.6C steel (wt.%) when the diffusible hydrogen content and flow stress were 0.36 wt.ppm and 1010 MPa, respectively.5)ε-martensitic transformation (FCC→HCP),6) deformation twinning,9) and strain aging10) are also known to assist the hydrogen embrittlement in Fe–Mn–C TWIP steels.
An important method for improving the resistance of Fe–Mn–C TWIP steels to hydrogen embrittlement is the addition of Al. For example, the Fe–18Mn–0.6C–1.5Al TWIP steel showed a higher resistance against the hydrogen embrittlement than that of the Al-free TWIP steels.3,8) The reason why the Al addition improved the hydrogen embrittlement has been discussed by considering various factors such as the formation of an Al2O3 layer13) on the surface and the change in the dislocation density and texture;8) however, it is still unclear. The Al effects for improving the resistance of Fe–Mn–C TWIP steels to the hydrogen embrittlement are assumed to be the reduction in the strength,4) suppression of twinning and ε-martensitic transformation due to the increase in stacking fault energy,14) the change in dislocation density and texture,8) and the inhibition of dynamic and static strain aging based on the abovementioned factors affecting the hydrogen embrittlement. In particular, the Al addition suppresses the dynamic and static strain aging by the increase in the activation energy for the carbon diffusion15) and the increase in the stacking fault energy.16) The suppression of dynamic strain aging that decreases the dislocation density8) and lowers the frequency of dislocation pinning by carbon resulting in a decrease in the material strength and work hardening rate15) and the inhibition of static strain aging by the Al addition that also decreases the material strength, are considered to reduce the hydrogen embrittlement susceptibility of the TWIP steels.
We evaluated the resistance of a Fe–22Mn–0.6C TWIP steel to hydrogen embrittlement by the tensile strain holding test under hydrogen charging after pre-straining in a previous work.10) A hydrogen-induced fracture was observed during the strain holding when the pre-strain rate before the strain holding was 1.7×10–5 s–1 and was suppressed by increasing the strain rate. The most important phenomenon in Fe–Mn–C austenitic steels affected by the change in strain rate has been found to be dynamic strain aging.16,17) Namely, the suppression of the hydrogen embrittlement by the increasing strain rate stems from the reduction in strengthening due to the inhibition of strain aging. The Al addition also inhibits the strain aging as mentioned above. Therefore, the resistance of Fe–Mn–C TWIP steels to hydrogen embrittlement evaluated by the strain holding tests with hydrogen charging must show Al content dependence. This observation would clarify the contribution of strain aging on the hydrogen embrittlement and would explain why the Al addition improves the resistance against hydrogen embrittlement.
The purposes of this study are (1) to evaluate the hydrogen embrittlement susceptibility of Al-added TWIP steels by the tensile strain holding test under hydrogen charging and (2) to clarify the Al effect on strain aging under loading. In actual automotive parts and practical experiments such as delayed fracture tests with cup specimens, the hydrogen-induced delayed fracture occurs after deformation and the subsequent hydrogen uptake with significant residual stress. Therefore, the tensile strain holding tests under hydrogen charging after pre-straining must reveal a mechanism of the actual hydrogen embrittlement of TWIP steels. In this paper, we report the correlation among Al content, strain aging, and occurrence of the hydrogen-induced fracture.
Fe–18Mn–0.6C–(0, 1.0, 1.5, 2.0)Al in wt.% steels were prepared by vacuum induction melting. The Fe–18Mn–0.6C–1.5Al steel is a typical TWIP steel that shows superior resistance against hydrogen embrittlement.3) The thickness of the steels was reduced from 60 to 2.6 mm by hot rolling at 1273 K and to 1.4 mm by cold rolling. Then, the steels were solution treated at 1073 K. All of the specimens were cut with spark erosion. Figure 1 shows that the microstructures of the Fe–18Mn–0.6C, Fe–18Mn–0.6C–1.0Al, Fe–18Mn–0.6C–1.5Al, and Fe–18Mn–0.6C–2.0Al steels have average grain sizes of 3.8, 4.4, 4.9, and 4.0 μm, respectively. The grain sizes include the annealing twin boundaries. Their thickness was reduced further by mechanical grinding to 0.3 mm to remove the layer affected by the solution treatment. The gauge dimension of the tensile specimens was 4.0 mmw × 0.3 mmt × 10 mml with a grip section on both ends to be fixed in an Instron type machine.
Optical micrographs of (a) Fe–18Mn–0.6C, (b) Fe–18Mn–0.6C–1.0Al, and (c) Fe–18Mn–0.6C–1.5Al, (d) Fe–18Mn–0.6C–2.0Al steels in the as-solution-treated condition.
Tensile tests including the process of strain holding were conducted at ambient temperature. As mentioned in the introduction, the actual hydrogen embrittlement of the TWIP steels occurs after deformation and the subsequent hydrogen uptake with residual stress. Therefore, hydrogen was introduced during the strain holding after pre-straining to 69%. The strains were determined by dividing the displacements by the initial gauge length. Figure 2(a) shows an example of the engineering stress-strain curve that contains the process of the strain holding under hydrogen charging in the Fe–18Mn–0.6C steel. The serrations are reported to arise from dynamic strain aging.16,17) The initial strain rate was 1.7×10–5 s–1. Subsequently, the strain was held at 69% plastic strain. The strain holding time was limited to 10 hours to avoid damaging the motor in the tensile machine. Hydrogen was introduced with a current density of 7 Am–2 in the 3% NaCl aqueous solution containing 3 g/L of NH4SCN immediately after the start of strain holding. A platinum wire was used as the counter electrode. When the hydrogen-induced fracture did not occur as in Fig. 2(a) the specimen was deformed again at a strain rate of 1.7×10–2 s–1 till fracture under hydrogen charging. Re-loading to fracture took less than 10 seconds.
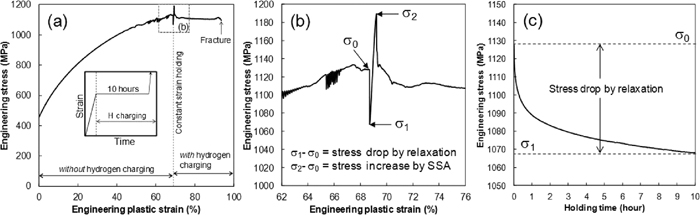
An example of stress-strain response at an initial strain rate of 1.7×10–5 s–1 in the Fe–18Mn–0.6C steel. (a) Engineering stress-strain curve. (b) Portion of Fig. 2(a) outlined by the dotted lines. (c) Engineering stress plotted against strain holding time. SSA: Static strain aging.
The hydrogen content was measured by means of a thermal desorption analysis (TDA) from room temperature to 550 K within 20 minutes after the specimen fractured. The heating rate was 200 K/h. The diffusible hydrogen content, which is defined as the hydrogen that diffuses at room temperature, was determined by measuring the cumulative desorbed hydrogen from ambient temperature to 523 K and is reported to play a key role in hydrogen embrittlement.18)
Figure 2(b) shows the magnified image of the part outlined by the square in Fig. 2(a). The stress drop by the strain holding and the stress increase by the re-loading can be observed. The stress drop is caused by the relaxation,19) and the stress increase is caused by the static strain aging.10,19) The magnitudes of the stress drop and the stress increase are defined as
| (1) |
| (2) |
As mentioned in the introduction, strain aging was reported to have an important effect on hydrogen embrittlement. Relaxation during the strain holding is also important. We have also conducted the abovementioned tensile tests with strain holding without hydrogen charging to investigate the effect of strain aging in the steels with various Al contents. The serrated flow increases considerably around the 69% plastic strain as shown in Fig. 2(b), disturbing the quantitative relationship among the stress change and various parameters. Therefore, the strain was held at a 49% plastic strain. Strain rate and strain holding time are known to be important factors affecting the relaxation and the static strain aging under loading when the strain rate sensitivity of the hydrogen embrittlement is discussed.10) Therefore, the dependences of the relaxation and the static strain aging on the strain rate and the strain holding time were also investigated. The strain rate of the pre-deformation was varied from 1.7×10–5 to 1.7×10–2 s–1. After strain holding at the 49% plastic strain, the specimens were deformed again at the same strain rate as that of the pre-deformation. These experiments were conducted once for each experimental condition.
Figure 3(a) shows the engineering stress-strain holding time curves under hydrogen charging for the Fe–18Mn–0.6C, Fe–18Mn–0.6C–1.0Al, Fe–18Mn–0.6C–1.5Al, and Fe–18Mn–0.6C–2.0Al steels pre-deformed to 69% plastic strain at a strain rate of 1.7×10–5 s–1. The curve for the Fe–22Mn–0.6C steel which was obtained by the same experimental condition in a previous study was also included as a reference.10) The Fe–22Mn–0.6C steel does not show any martensite even after fracture and has a lower flow stress than that of the Fe–18Mn–0.6C steel.10) Although the Fe–22Mn–0.6C steel showed a hydrogen-induced fracture, none of the present steels showed the fracture regardless of the Al content during the strain holding after the pre-deformation at the strain rate of 1.7×10–5 s–1. Delayed fracture tests with cup specimens also showed that the hydrogen embrittlement susceptibility of the Fe–22Mn–0.6C steel is higher than that of the Fe–18Mn–0.6C steel.3,20) In contrast, the Fe–18Mn–0.6C steel pre-deformed to 69% plastic strain at a lower strain rate of 1.7×10–6 s–1 showed the hydrogen-induced fracture during the strain holding under hydrogen charging as shown in Fig. 3(b). The stress gradually decreased due to the relaxation phenomenon, and the fracture finally occurred at the holding time of about 8 hours. Figures 3(a) and 3(b) show that the 69% flow stress increased with decreasing strain rate from 1.7×10–5 to 1.7×10–6 s–1. The increase in flow stress is the so-called negative strain rate sensitivity that is caused by the promotion of the dynamic strain aging.16,17)
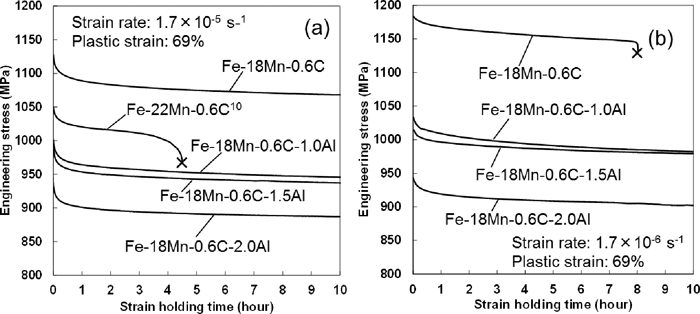
Change in engineering stress during strain holding after the pre-deformation at strain rates of (a) 1.7×10–5 and (b) 1.7×10–6 s–1 in the Fe–18Mn–0.6C, Fe–18Mn–0.6C–1.0Al, Fe–18Mn–0.6C–1.5Al, and Fe–18Mn–0.6C–2.0Al steels. The strain was held at 69%. Hydrogen was introduced at the current density of 7 Am–2. The result of the Fe–22Mn–0.6C steel was obtained from our previous paper.10)
The fractograph in Fig. 4 shows an intergranular fracture. A premature fracture due to strain localization can be considered as a possible mechanism of hydrogen-related degradation of mechanical properties when dynamic strain aging occurs. When the strain localization causes the premature fracture, a fully ductile fracture is observed. However, the premature fracture can be ruled out as the crack initiation mechanism of the steel in Fig. 4 since an intergranular fracture was observed. The effect of Al on the reduction of hydrogen embrittlement susceptibility of the Fe–18Mn–0.6C–based steels at the initial strain rate of 1.7×10–6 s–1 shown in Fig. 3(b) has been reported to be associated with the suppression of the formation of ε-martensite.20) However, Fig. 3(a) shows that the hydrogen embrittlement susceptibility of the Fe–18Mn–0.6C steel was lower than that of the Fe–22Mn–0.6C steel that did not show martensitic transformation even after fracture in a tensile test without hydrogen charging.21) On the other hand, the phase stability of austenite in the Fe–22Mn–0.6C steel was higher than that in the Fe–18Mn–0.6C steel, since the increase in Mn content suppressed the formation of martensite.22) These facts indicate that Al has other effects on the hydrogen embrittlement in addition to its influence on suppressing the martensite formation.
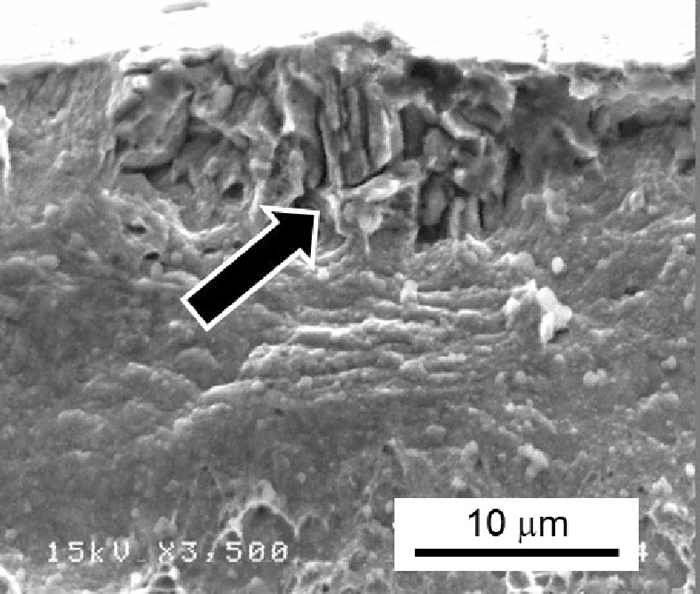
Fractograph of the Fe–18Mn–0.6C steel showing the intergranularly fractured part as indicated by the arrow. The specimen was fractured during the strain holding at 69% plastic strain after the pre-deformation at the strain rate of 1.7×10–6 s–1 as shown in Fig. 3(b).
Figure 5 shows the thermal hydrogen desorption rate curves after the abovementioned experiments. The diffusible hydrogen content was determined from the TDA results. The peak at around 400 K was reported to correspond to the diffusible hydrogen in the Fe–Mn–C austenitic steel.11)Figure 6 shows the diffusible hydrogen content plotted against Al content. The Al-containing steels showed almost the same or more diffusible hydrogen content than that of the Al-free steel at strain rates of both 1.7×10–5 and 1.7×10–6 s–1, although the Al content dependence of diffusible hydrogen content is not simple. Ryu et al. also reported23) that the diffusible hydrogen content of the Fe–18Mn–0.6C–1.5Al steel is much higher than that of the Fe–18Mn–0.6C steel. On the contrary, the diffusible hydrogen content of TWIP steels has also been reported to decrease with increasing Al content.8,12,13)
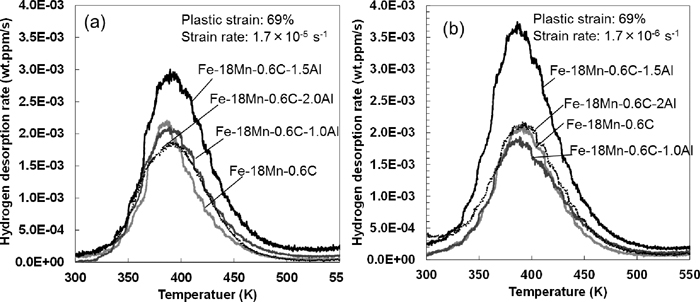
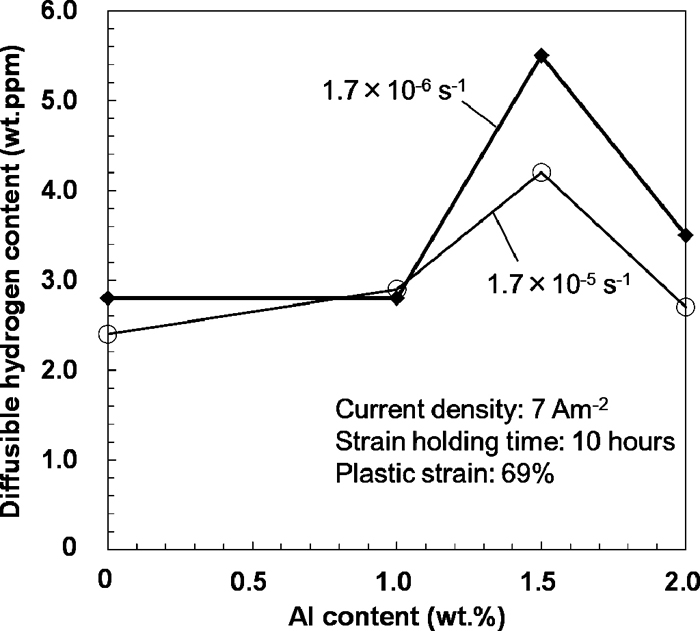
Diffusible hydrogen content estimated from Fig. 5.
Carbon segregation to the dislocations by static strain aging is known to decrease the number of hydrogen trapping sites due to site competition, resulting in a decrease in the diffusible hydrogen content.24,25,26) Toji et al.25) investigated the effects of low temperature heat treatment on the hydrogen uptake in ferritic steels. They showed that the hydrogen uptake was suppressed by increasing the solute carbon content when the steels were heat treated at low temperature. They claimed that the suppression occurred since the carbon atoms segregated and occupied the hydrogen trap sites, which are dislocations. Since Al addition can suppress static strain aging due to an increase in the activation energy for carbon diffusion, it is expected to hinder the carbon atoms from occupying the sites acting as hydrogen traps and to increase the diffusible hydrogen content from the viewpoint of static strain aging. Note that the effect of dynamic strain aging is completely different from the effect of static strain aging, namely, dynamic strain aging increases the dislocation density to provide further plastic strain,8,27) although carbon segregation to dislocation also occurs by dynamic strain aging. Hence, there are three Al effects affecting the diffusible hydrogen content in the present experiment: the formation of an Al2O3 layer on the surface,13) an increase in dislocation density due to dynamic strain aging,8) and the decrease in hydrogen trapping sites due to static strain aging. The first and second effects decrease the diffusible hydrogen content, while the third effect increases it. The combination of these effects complicates the Al content dependence of diffusible hydrogen content as shown in Fig. 6. The significance of the Al effect on static strain aging will be discussed in the next section. Nonetheless, these facts indicate that the suppression of the present hydrogen embrittlement by the Al addition and by the increase in strain rate is not attributed to the decrease in diffusible hydrogen content.
Consequently, the Al effect on strain aging can be considered as the factor affecting the hydrogen embrittlement. Figure 7 shows the engineering stress-strain curves at the initial strain rate of 1.7×10–5 and 1.7×10–6 s–1 in the three steels. The flow stresses decrease with increasing Al content as well as with strain rate. Additionally, the serrated flow arising from the dynamic strain aging is suppressed by the Al addition at both strain rates. Namely, the dynamic strain aging was confirmed to be suppressed by increasing the Al content as well as the strain rate. Figure 7(c) shows the Al content dependence of 69% flow stress change by decreasing the strain rate from 1.7×10–5 to 1.7×10–6 s–1. The negative strain rate sensitivity was observed particularly in the Al-free steel. Since a larger amount of diffusible hydrogen is required for hydrogen-induced fracture when flow stress decreases,8,11) the decrease in flow stress, i.e. material softening, by increasing the strain rate and Al content must decrease the hydrogen embrittlement susceptibility.
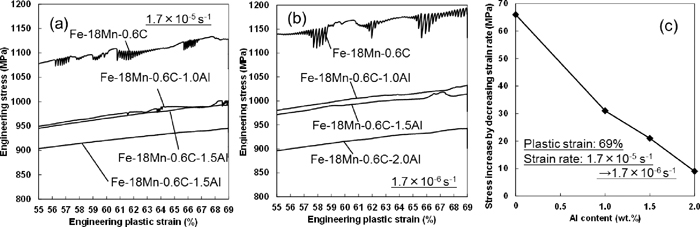
Portions of engineering stress-strain curves at the initial strain rates of (a) 1.7×10–5 and (b) 1.7×10–6 s–1. (c) 69% flow stress change by decreasing strain rate from 1.7×10–5 to 1.7×10–6 s–1.
Since the addition of Al suppressed dynamic strain aging, it would also influence the static strain aging. The stress drop by the relaxation and the strengthening by the static strain aging are important factors affecting the hydrogen embrittlement property.10) The static strain aging was reported to occur significantly under loading,19) as confirmed in Fig. 1. In this section, we show the effects of Al on the relaxation and the static strain aging under loading.
Figure 8 shows the Al content dependence of the stress drop due to the relaxation. The stress drop was measured by means of the strain holding of the specimen pre-strained to 49% plastic strain at a pre-strain rate of 1.7×10–2 s–1. The magnitude of the stress drop was estimated using Eq. (1). It decreased slightly by increasing the Al content. Thus, the Al effect suppressing the hydrogen embrittlement of the Fe–Mn–C TWIP steels does not result from the relaxation behaviour.
The magnitude of stress drop due to the relaxation and its Al content dependence. The values for each holding time were obtained from different tests.
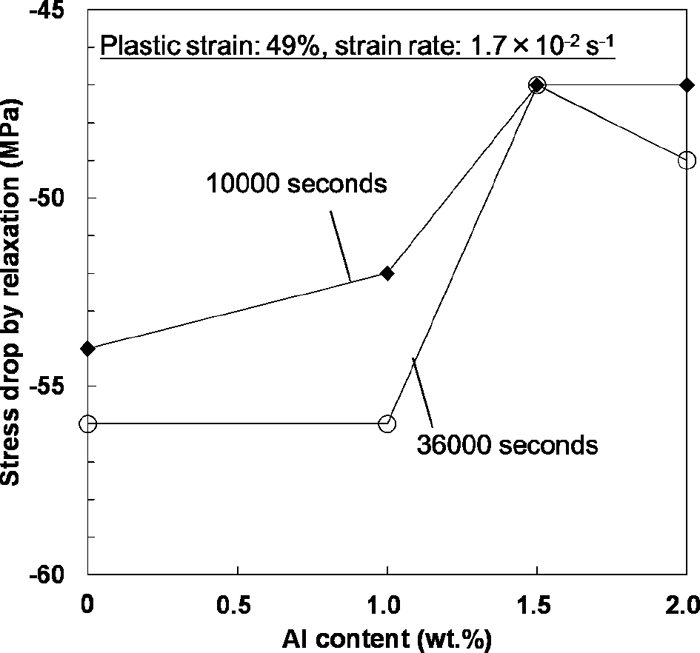
Figure 9 shows the Al effect on static strain aging under loading at a constant strain. The magnitude of strengthening by the static strain aging was estimated using Eq. (2). The plastic strain was 49%, and the strain rate was 1.7×10–2 s–1. The specimen was deformed again at a strain rate of 1.7× 10–2 s–1 after the strain holding. The magnitude of strengthening by the static strain aging increased linearly with logarithmic aging time as shown in Fig. 9(a), and decreased with increasing Al content as shown in Fig. 9(b). Since the Al addition decreased the slope in Fig. 9(b), the effect of Al on static strain aging increased considerably as the aging time increased. When we consider the effect of static strain aging in Fe–Mn–C austenitic steels, the effect of strain rate of pre-straining must also be examined.10) Figure 10 shows the strain rate effect on static strain aging. The magnitude of strengthening by the static strain aging decreased linearly with decreasing logarithmic strain rate. The same trend was also reported in a Fe–22Mn–0.6C TWIP steel from a previous study.10) The slope of the approximate line did not change with the Al addition as shown in Fig. 10.
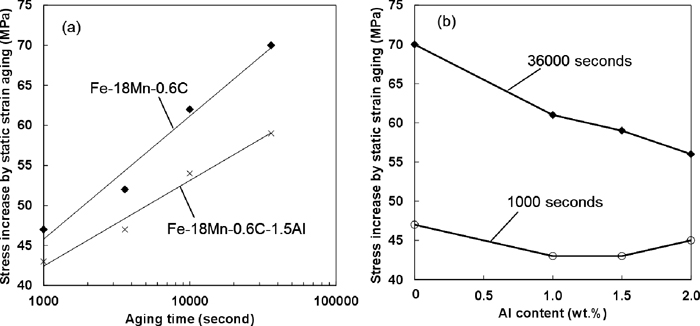
(a) Stress increase by the static strain aging under loading plotted against aging time. (b) Al effect on the stress increase after strain holding for 1000 and 36000 seconds.
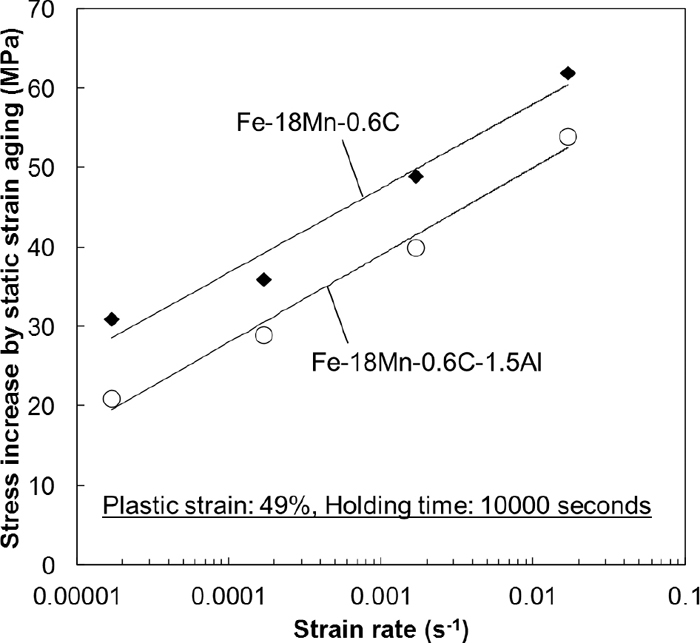
Strain rate dependence of the stress increase due to the static strain aging. The Al addition did not change the slope significantly.
From the viewpoint of the static strain aging, the Al effect on the hydrogen embrittlement should be discussed by considering the increase in materials strength by aging. Note that the effects of external stress and materials strength must be considered separately for the present case. Hydrogen embrittlement susceptibility is known to increase with increasing material strength.28,29,30,31) The critical stress for hydrogen-induced fracture can be much lower than the yield strength in some high strength steels.31) As schematically shown in Fig. 11, the external stress decreases with time by relaxation, while the materials strength increases with time due to the static strain aging during the strain holding. Although the external stress decreases, the hydrogen-induced fracture can occur during the strain holding when the materials strength reaches a sufficiently-high value.
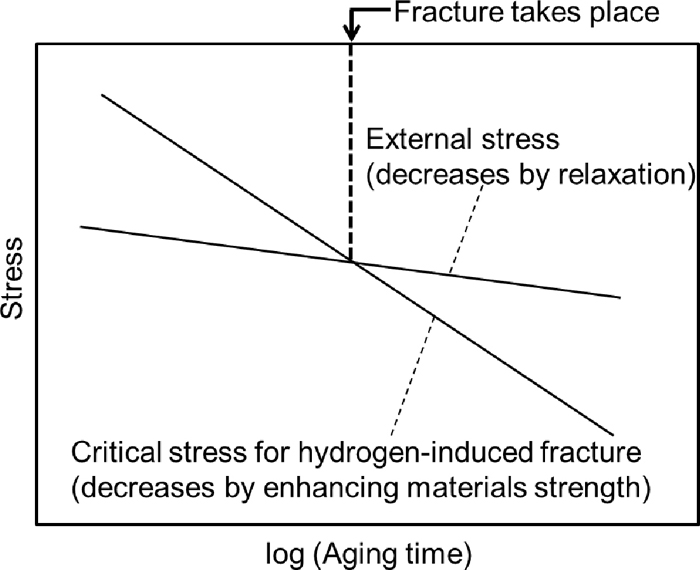
Relationship among external stress, critical stress for hydrogen-induced fracture, and occurrence of fracture at a constant hydrogen content. The material strength increases by static strain aging during holding at a fixed strain under loading, decreasing the critical stress for hydrogen-induced fracture.
We propose a possible mechanism of the material strengthening effect on the hydrogen embrittlement with strain aging. First, the hydrogen-induced fracture process is considered. The hydrogen-related cracks of TWIP steels are initiated at the grain boundary triple junctions or the grain and twin boundaries intercepting the deformation twins.32) These brittle cracks nucleate when hydrogen localizes sufficiently to the specific sites. The brittle cracks are considered to nucleate before fracture, since a considerable number of sub-cracks are observed around the fractured parts in both the Al-added7) and the Al-free steels.9,32) Then, the brittle cracks grow till they reach a critical size for fracture. Namely, there are two types of factors that determine the critical stress for hydrogen-induced fracture: the factors affecting crack initiation and those influencing crack propagation. The effect of strain aging would be a factor for crack propagation. Strain aging hardens the steel through dislocation pinning by carbon segregation; however, once dislocation depinning occurs, it results in a local material softening. Stress concentration existing at the crack tip can cause the local dislocation depinning when there is a sufficient amount of pinned dislocations due to dynamic and static strain aging. Therefore, strain aging promotes the crack propagation of a given crack via the local softening and decreases the critical stress for hydrogen-induced fracture. This mechanism can be used to explain some conventional results on the Al effect on hydrogen embrittlement susceptibility of TWIP steels as follows. The Al effect suppressing the hydrogen embrittlement was observed clearly in the delayed fracture tests with deep-drawn cup specimens20) and in the present work. In contrast, the tensile tests under hydrogen charging and the tensile tests after hydrogen-pre-charging did not show a clear difference in the hydrogen-affected tensile properties between the Al-added and Al-free steels, although the elongation of the Al-added steels was slightly larger than that of the Al-free steel.2,23) Static strain aging affects only the first group of the tests (i.e. delayed fracture test and the present work), indicating that the Al effect becomes remarkable when the hydrogen embrittlement properties are evaluated by the experiments. In contrast, the crack propagation in the tensile tests during hydrogen charging must be controlled by the hydrogen uptake after the crack nucleation, indicating that Al cannot contribute to a reduction in hydrogen embrittlement susceptibility in terms of strain aging.
Figure 12 shows the correlation among Al content, magnitude of strengthening by the static strain aging, and hydrogen embrittlement susceptibility by combining the strengthening and Al effects on static strain aging under loading. The Al addition decreases the flow stress and the material strength due to the suppression of deformation twinning, dynamic strain aging, and static strain aging. By assuming that there is a critical material strength for the hydrogen-induced fracture as indicated in this figure, the time to reach the critical material strength increases with increasing Al content. In particular, the suppression of the static strain aging is crucially important when the aging time under stress is long, since the Al addition decreases the rate of strengthening with time, and the magnitude of strengthening by the static strain aging increases linearly with logarithmic time unless the effect of the static strain aging is saturated. Hence, the decrease in the material strengthening rate per aging time by the Al addition delays the time to fracture exponentially and improves the resistance of the steels against hydrogen embrittlement.
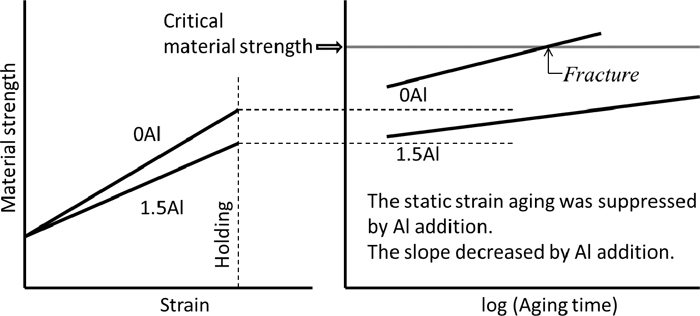
A schematic for how the Al addition contributes to the hydrogen embrittlement, from the viewpoint of strain aging. The critical material strength refers to the strength for fracture at a given condition of external stress and hydrogen content.
The Al content and strain rate dependence of the hydrogen embrittlement susceptibility was investigated by the strain holding tests under hydrogen charging in Fe–18Mn–0.6C, Fe–18Mn–0.6C–1.0Al, and Fe–18Mn–0.6C–1.5Al TWIP steels. The hydrogen embrittlement susceptibility decreased by increasing the Al content and strain rate of the pre-deformed steels. In this study, static strain aging under loading was suppressed by the Al addition and dynamic strain aging during pre-deformation was suppressed by increasing the Al content and strain rate, and these factors influenced the hydrogen embrittlement. From the viewpoint of strain aging, the strain rate effect on the hydrogen embrittlement is associated with the negative strain rate sensitivity of flow stress due to the suppression of dynamic strain aging.
M. K. acknowledges the Research Fellowship of NIMS Junior Researcher (2009–2010) and the Japan Society for the Promotion of Science for Young Scientists (2011). POSCO supported this work through the provision of the samples and funding.