2013 Volume 53 Issue 8 Pages 1341-1349
2013 Volume 53 Issue 8 Pages 1341-1349
The addition of MgO to iron ore pellets is known to beneficially influences many high temperature reduction properties such as reducibility and swelling. When the pellet is metallized, MgO dissolved in the wustite concentrates in the unmetallized part, which is why MgO-levels much higher than the average concentration could be expected locally. In this work the impact of the elevated MgO-content on the reduction at 1000–1300°C was studied by SEM-EDS. The MgO content in the pellet was also varied by additions of a), highly reactive olivine b) unreactive olivine c) combined addition of reactive olivine and fine quartzite and d) combined addition of unreactive olivine and fine quartzite. Two cases of metallization were observed 1) a gradual reduction front with only moderate magnesium levels and 2) a sharp reduction front with strongly elevated magnesium levels before the metal front. The samples with added quartzite reduced a little better at 1100°C, compared to those with only olivine, but apart from that, reduction was not affected much by the additives in the range 1000–1200°C. The greatest difference in reduction degree appeared at 1300°C where a metal skin formed in most samples, hindering further reduction. At this temperature, the sample with addition of only reactive olivine had superior reducibility due to a porous morphology of the iron being mantained throughout the experiment.
Recently, environmental concerns have placed increasing pressure on the CO2-intensive iron and steel industries to further optimize operation towards higher productivity and lower consumption of reducing agents. At the same time, high demand for iron ore has made new iron ores with lower grades and more complex chemistries viable in production. In Scandinavia the main iron-bearing material is magnetite based pellets with olivine, which allows operation with very low slag volumes and hence lower fuel rate.1,2) In these pellets both the MgO and SiO2 of the olivine as well as the elements of the gangue of the ore, i.e. Si, Mg, Ca and Al, interact with the iron oxides to give the finished pellet product its final properties in the blast furnace. Tailoring the additive mix to be used for a certain iron ore for optimal product properties therefore requires detailed knowledge of the mechanisms by which these elements dissolve and react in the pellet.
The many beneficial effects of MgO additions during reduction have been summarized in a previous study,3) the most important one being the enhanced high-temperature properties due to the dissolution of magnesium into the wustite and the slag. However, during oxidation, the olivine decomposes partly, so that MgO dissolves in the magnetite forming magnesioferrite.4,5) As the magnetite oxidizes, the original ore particles turn into parts oxidized into pure hematite and parts of magnesioferrite.4) The boundaries between these two phases in the same original magnetite particle are cohesive and extensive, but have been reported to open up in wide gaps during low-temperature reduction.6) It is desirable to avoid the low-temperature impact and still keep the high-temperature benefits, and therefore it has been recommended that a course unreactive olivine should be used to minimize the magnesioferrite formation during the oxidation process6,7) However, the olivine itself is very unreactive until above 1150°C in reducing atmosphere; still, olivine is known to possitively influence both swelling and reducibility in the temperature range of 900–1100°C. Therefore, a series of studies was started to clarify the reaction mechanisms for MgO in pellets during reduction.
Previous studies showed that diffusion of magnesium from the magnesioferrite to the magnetite and wustite forming commenced at about 800°C8) during reduction, and that further dissolution from the wusitite into the silicate slag occured at 1000°C.3) Another recent study by the authors9) showed that a fine reactive olivine, converting completely into magnesioferrite during oxidation, caused the slag to separate into fayalite and an alkali-rich remnant. When a course unreactive olivine was used, very little magnesioferrite formed during oxidation, and then the separation of the slag occurred first at 1200°C when the unreacted olivine cores started to dissolve in reducing atmosphere. These results indicate that the magnesium of the magnesioferrite forming during oxidation is also the portion responsible for the beneficial effect on important phenomena such as reducibility and swelling until above 1100°C.
In the current study the impact of the metallic iron formation on the pellet was studied, during reduction in the temperature range of 1000–1300°C. The dissolution of all magnesium in a 2.5% olivine addition into the wustite and slag raises the melting point of the fayalitic slag from about 1200 to 1250°C compared to fayalite with no magnesium, according to thermodynamic calculations. However, in a real ironmaking process several of the different hematite-magnetite-wustite-iron reduction steps may take place simultaneously at different locations along the pellet radius. As the wustite transforms into iron, more and more magnesium will concentrate into the remaining wustite, in front of the growing iron layer, which could significantly raise the local magnesium concentrations and thereby also strongly influence the high-temperature properties. The samples were the same as those tested during reducion to wustite:9) pellets with fine and course olivine, with and without additional fine quartzite(< 20 μm).
In this study, micro-balled pellets were prepared with a very fine olivine as well as with a very coarse one to facilitate high and low reactivity during induration. Cases with extra silica enabling early slag formation, sintering and good contact in the sample were also included. The pellets were first oxidized in air at 1250°C, and then reduced at 1000, 1100, 1200 and 1300°C. Both the oxidation and the reduction were done isothermally in a tubular furnace, see Fig. 1. The gas used for the reduction tests was 100% CO, which, thermodynamically gives reduction to metallic iron at all considered temperatures. The temperature was recorded with a thermocouple located 3 cm under the sample. The tubular furnace was preheated to the desired temperature and the samples were then inserted. Three pellets were tested for each sample. The sample weight gain or weight loss was continuously recorded by a thermo-balance during two hour-long oxidation and reduction periods, after which the samples were lifted out. The oxidized samples were cooled in room atmosphere, whereas the reduced samples were quickly moved over into a cooling chamber and immediately cooled in a flow of nitrogen gas for 20 min, then in room atmosphere.

Schematic view of the experimental apparatus used for the reduction tests.
The pellets were made from a magnetite concentrate to which the additive minerals olivine and quartzite were added. 0.5% bentonite was also added to the concentrate for balling purposes. The olivine was sieved to produce one coarse (> 108 μm) and one fine fraction (< 38 μm). The fine fraction was wet-sieved followed by filtering and drying. The size distribution was then measured with a CILAS 1064, which gave a mean particle diameter of 184 μm and 90% > 78 μm for the coarse fraction and a mean particle diameter of 15.74 μm and 90% < 33 μm for the fine fraction. Pellets with fine quartzite were also prepared to provide larger amounts of slag and improved contact in the pellets. The quartzite was wet-sieved to separate the < 20 μm fraction, which was then filtered and dried. The size distribution was measured with a CILAS 1064, giving 16 μm in mean particle diameter and 90% < 36 μm. A reference pellet, in which the only additive was 0.5% bentonite, was also prepared for comparison. The pellets were micro-balled at LKAB and sieved to collect the 9–12.5 mm fraction. The average pellet weight was 3.3 g after drying. Table 1 displays the specification of the different pellets tested. Table 2 provides a complete raw material specification, as well as calculated chemical analysis of the finished pellets.
| Olivine | Quartzite | Bentonite | Test temp. | |
|---|---|---|---|---|
| Sample 1 (S.1) | 2.5 mass% > 108 μm | None | 0.5 mass% | 1000–1300°C |
| Sample 2 (S.2) | 2.5 mass% < 38 μm | None | 0.5 mass% | 1000–1300°C |
| Sample 3 (S.3) | 2.5 mass% > 108 μm | 3.0 mass%< 20 μm | 0.5 mass% | 1000–1300°C |
| Sample 4 (S.4) | 2.5 mass% < 38 μm | 3.0 mass%< 20 μm | 0.5 mass% | 1000–1300°C |
| Sample 5 (S.5) (reference) | None | None | 0.5 mass% | 1000–1200°C |
| Fe | FeO | SiO2 | MgO | CaO | Σ Na2O + K2O | |
|---|---|---|---|---|---|---|
| Iron ore conc. | 71.26 | 0.56 | 0.17 | 0.16 | 0.08 | |
| Olivine coarse | 5.19 | 40.96 | 44.93 | 0.44 | 0.173 | |
| Olivine fine | 5.04 | 38.80 | 45.16 | 0.13 | 0.14 | |
| Quartzite | 0.43 | 98.15 | 0.04 | 0.05 | 0.228 | |
| Bentonite | 3.83 | 51.46 | 3.87 | 7.16 | 3.56 | |
| Samples 1(S.1) calculated | 96.10 | 2.14 | 1.45 | 0.18 | 0.13 | |
| Samples 2(S.2) calculated | 92.95 | 5.29 | 1.44 | 0.18 | 0.13 | |
| Samples 3(S.3) calculated | 96.16 | 2.08 | 1.46 | 0.18 | 0.13 | |
| Samples 4(S.4) calculated | 93.01 | 5.24 | 1.45 | 0.17 | 0.13 | |
| Sample 5(S. 5)(reference) | 98.42 | 1.05 | 0.24 | 0.18 | 0.13 | |
| calculated |
The samples were characterized before and after reduction by a scanning electron microscope (SEM) with an energy dispersive attachment (EDS). This was facilitated by a Zeiss Merlin with an Oxford Instruments X-Max 50 mm2 detector, and a FEI company Magellan 400 with an Oxford X-max 80 mm2 detector . The quantitative analysis was done with ZAF-correction and calibrated against Copper. The pellets were mounted in epoxy resin and polished down to 1 μm- fineness with diamond pastes before carbon coating. As chemistry and morphology in the pellets were found to vary across the pellet radius, images and point analyses were taken both in the pellet periphery and in the core to obtain a representative view of the pellets from each experiment. In each of the chosen areas for analysis typically 12–40 chemical point analyses were taken to gain an overview of the composition in the phases present.
2.3. Thermodynamic CalculationsThermodynamic calculations were made in Factsage 6.210) using compound database FS53base.cdb, FToxid53base.cdb and solution databases FToxid53soln.sda. FToxid-slagA, FToxidOlivA, FToxid-cPyrA, FToxid-oPyr, FToxid-pPyrA and FToxid-MeO_A. During calculation, FS53base.cdb was suppressed contra FToxid53base.cdb to exclude duplications in the data set. In the study the experimental results were compared with calculated ternary phase diagrams. The best fit to the experiments was obtained by using a temperature 50°C higher than the experimental temperature. Therefore, the phase diagrams used to explain the samples are always calculated 50°C higher than the actual temperature of interest. As an example, the phased diagrams calculated at 1150 and 1250°C are used to display the Mg-equilibriums in the samples reduced at 1100 and 1200°C.
2.4. NomenclatureThe results from this study of reduction to metallic iron will be compared with results from a previous study with the same experiments done on similar samples, but with reduction only to wustite. For convenience this study will hereafter be labelled RedWUS.9)
In the oxidizing atmosphere the coarse olivine particles have decomposed along the particle periphery to form magnesioferrite crystals and vitreous silica, see Fig. 2(a). The fine olivine particles have reacted completely, so it is very difficult to find the original particle sites. No intact olivine cores can be found in the samples with fine olivine (S.2 and S. 4), see Fig. 3, and the appearance is therefore more homogenous than that of the samples with coarser olivine (S.1 and S. 3). At the same time as the olivine particles have dissolved, magnesium has been released and diffused out to form magnesioferrite amongst the surrounding hematite. The presence of this magnesioferrite is different in the shell and core of the sample with coarse olivine, whereas it is very evenly distributed in the pellet with fine olivine. In the case of coarse olivine, the magnesioferrite is associated with the olivine particles in the shell, whereas it is more randomly spread out among the secondary hematite in the centre. The mechanism for formation of magnesioferrite during oxidation in olivine pellets has been described elsewhere.4)

SEM-BSC image from the central areas of a pellet with coarse olivine after oxidation at 1250°C. Mg-Ferr. = Magnesioferrite, Corona = Reaction corona of vitreous silica and magnesioferrite, P = pore.

SEM-BSC image from the central areas of a pellet with fine olivine pellet after oxidation at 1250°C. Mg-Ferr. = Magnesioferrite, P = pore.
In the pellets with quartzite addition, quite intact 10–30 μm quartzite particles are smoothly distributed in the samples. These particles, especially the ones smaller than 10 μm, have sintered to stick to the nearest hematite particles. Absence of really small quartzite particles is believed to be due to these being more reactive and hence having dissolved in the slag. Other than that, both the fine and the course olivine samples appear similar to those without quartzite addition.
3.2. ReductionTable 3 summarizes the main reactions occurring during reduction of the samples at the four test temperatures. In oxidizing conditions hematite is in equilibrium with magnesioferrite (state 1 in Fig. 4). When the oxidized sample is reduced, equilibrium conditions are shifted along the path marked out in the Fe2O3–Fe–MgO–system, as seen in Fig. 4. In the reduction tests, the stable iron oxide is shifted from hematite via magnetite and wustite to metallic iron(state 2) in Fig. 4. The magnesioferrite in the oxidized sample reduces also to magnesiowustite, and then to metallic iron. In the last step the magnesium that does not dissolve in the iron is expelled and must concentrate in remaining wustite and in the slag. As wustite (FeO) forms a complete series of solid solutions with MgO, the magnesium of the magnesiowustite can now freely dissolve in the wustite layer.
| Temp, °C | Stable phase | Observed reaction | |
|---|---|---|---|
| a) | 1000 | Fe | Advancing gradual metal front, wustite particles + slag inclusions In course-olivine sample also almost intact olivine particles |
| b) | 1100 | Fe | Advancing gradual metal front, wustite particles + slag inclusions In course-olivine sample also almost intact olivine particles |
| c) | 1200 | Fe | Advancing gradual metal front, remaining wustite particles rounded if in contact with slag. Fayalitic slagphase with vitreous alkali rich inclusions. Some very large intact olivine particles still remaining in course olivine-samples |
| d) | 1300 | Fe | No olivine particles remaining. Two cases, 1) Metal shell closing the structure for further reduction in S.1 , S.3 and S.4. Thick layer of slag between the iron shell and main body of spherical wustite particles surrounded by olivine/alkali slag phase. 2) Porous metal formation in S.2 and S.5, with almost 100% conversion to iron . |

Phase diagram for the system MgO–Fe–Fe2O3 obtained using Factsage. The compositions for the equilibrium systems at oxidation, and at reduction to iron are marked out in the diagram as points 1 and 2, respectively.
The reduction curves in Fig. 5 show that the reduction in general is higher, the higher the temperature, until 1200°C. But at 1300°C the reduction is much lower in all samples except in that with fine olivine (S. 2) and in the reference (S. 5). In S. 1, S. 3 and S. 4 the melting of the slag components causes the metal to sinter into a dense shell, which introduces a significant obstacle for further reduction at 1300°C. At 1100°C, the samples with added quartzite reduce slightly more slowly than those with only olivine. At 1200°C and 1300°C sample S. 3 shows the slowest reduction behaviour.

Relative loss of weight during reduction for S. 1–S. 5 at 1000–1300°C.
Results presented in Fig. 6 show that the swelling was greatest for the reference over all temperatures, and that the different additives helped to reduce expansion or even cause shrinking at the highest temperature. The greatest swelling is at 1100–1200°C for S. 1–S. 4, whereas the reference (S.5) has the highest swelling in the beginning, followed by a decrease the higher the temperature gets. The relative order of the swelling between S. 1–S. 4 changes between 1100° and 1200°C. At 1000 and 1100°C, S. 1 swells the most, S. 2 and S. 3 are intermediate and S. 4 swells the least. At 1200 and 1300°C there is not much of change for S. 1, S. 2 and S. 5, but S. 3 and S. 4 clearly shrinks here.
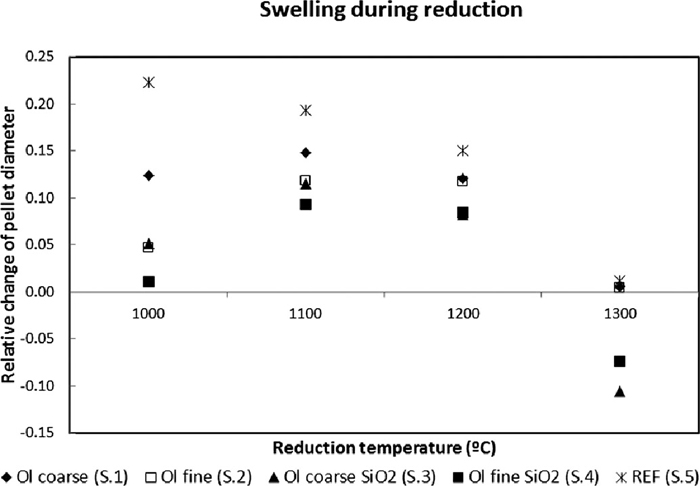
Swelling measured as relative increase in diameter during reduction for S. 1–S. 5 at 1000–1300°C.
In the samples reduced at 1000 and 1100°C the iron formation is complete in the peripheral region, and then decreases gradually towards the core. Due to this gradual increase of iron, no front with highly elevated magnesium levels occurred at these temperatures. However, in most samples the iron formation has occurred topo-chemically on larger particles in the periphery, which has led to very high magnesium concentrations, up to 50% MgO, in wustite entrapped in the centre of metallized particles. This is true especially in S. 1 and S. 2 reduced at 1000–1100°C. Inclusions next to olivine particles in S. 1 and S. 3 also hold high MgO contents, even up to 80%, after reduction at 1000°C.
At 1000°C, in the peripheral region, the iron has formed topochemically to enclose a core of wustite in all particles. But the particles in the samples S. 1, S. 2 and S. 4 have also incorporated significant amounts of pores and defects, which has led to a higher conversion to iron in each particle, compare Figs. 7 and 8. In the centre of the pellets the greatest impact seems to come from the presence of quartzite. Iron formation in S. 1 and S. 2 occurs non-topochemically, whereas that of S. 3 and S. 4 occurs topochemically. The quartzite particles in S. 3 and S. 4 have sintered to bind to neighbouring wustite particles; apart from that, they are unaffected at this temperature.

Particles with large amounts of pores and defects in the periphery of S. 2 after isothermal reduction at 1000°C. P = Pore.

Particles with less pores and defects in the periphery of S. 3 after isothermal reduction at 1000°C. P = Pore.
At 1100°C the porosities of the S. 1–S. 4 now reflect the same trend as the swelling properties with the highest porostity for S. 1 and lowest for S. 4. Again, however, S. 2 and S. 4 show a higher microporosity in particles because of irregular shapes and defects. In the centre, iron formation has occurred non-topochemically in all samples. The quartzite particles have melted in both S. 3 and S. 4 to form fayalite at 1100°C.
3.5.2. 1200°CAfter reduction at 1200°C the metallization is high and the iron formation has reached the centre in all samples except for in sample 3 which has the lowest reduction degree. The fully metallized part reaches down to a ~3.5 mm depth in the pellets and beyond this depth the presence of unreduced wustite increases gradually along the radius to the centres. S. 1–S. 4 consist mostly of wustite in the centre. Even here, the absence of a distinct reduction front leads to only moderate Mg-levels in the samples. Sintering has occurred mainly at locations in contact with the slag, which has left many macropores in the samples in locations where no liquid slag could facilitate densification. However, in S. 3 the core has sintered more than the other samples to aquire the structure typical for the samples reduced at 1300°C, which is rounded wustite spheres completely surrounded by a slag matrix, and only few remaining macropores.
3.5.3. 1300°CAfter reduction at 1300°C, a dense iron shell has formed in S. 1, S. 3 and S. 4, and this metal shell, with a thickness of 100–250 μm, has stopped further reduction in the samples, see Fig. 9. Inside the metal shell is a 200–550 μm-thick layer of fayalitic slag with small wustite inclusions forming a pattern resembling crystals forming from a precipitating liquid. The inner structure of wustite spheres and molten slag is very similar to that observed in RedWUS.9) In the reference, the wustite has reduced to iron throughout the pellet and also in S. 2 the reduction has been almost complete in two of the three pellets in the sample. Wustite inclusions in the metallized particle in the pellet centre reached levels of up to 70% MgO. The iron front in the third pellet of S. 2 was quite narrow, and high MgO levels, 10–15%, were found also just before this front.

A dense metallic shell and a zone of crystallized liquid slag around the unreduced wustite in S. 1 after isothermal reduction at 1300°C.
The magnesium concentration in the slag and wustite in the centre of S. 1–S. 4 is displayed in Figs. 10 and 11. Also, for comparison, the data of the samples reduced only to wustite at 1000–1200°C9) is included in pale grey in the diagram. Overall the trends for the change in magnesium for the samples resemble those for reduction only to wustite, even though the magnesium levels are generally slightly higher in the current study. A few remarks may be made:

The background magnesium levels recorded in the wustite of the tested pellets at 1000–1300°C. Concentrations calculated for reduction to wustite (RedWUS)9) superimposed in pale gray.

The background magnesium levels recorded in the slag of the tested pellets at 1000–1300°C. Concentrations calculated for reduction to wustite (RedWUS)9) superimposed in pale gray. *In S. 1 (1000–1100°C) the slag is mostly of the vitreous type, i.e. fayalite crystals have not precipitated.
In the course olivine S. 1 and S. 3 the magnesium level appears not to be affected significantly by the presence of iron. The magnesium from the olivine has not dissolved much during oxidation, which is why the main increase appears at 1200°C when intact olivine particles begin to dissolve in reducing atmosphere. Sample 4 has a slightly lower magnesium content at 1000°C compared to when reduced only to wustite, but at all the other temperatures the Mg-level is higher after reduction including metallization.
To describe the magnesium diffusion behaviour, four chosen cases from the fine olivine samples (S. 1 and S. 2) were selected and presented in detail.
3.6.1. 1100°CThe magnesium content in the fayalitic slagphase and the wustite in the pellet centre was recorded by SEM-EDS and the compositions plotted in the phase diagram in Fig. 12. The relation between the wustite and slag matches very well with the phase diagram tie-line in the figure. An increasing Mg-content means that the total composition shifts left along the horizontal arrow in the figure. It is clear that the proceeding metallization has caused the overall composition to enrich in magnesium compared to the composition of the sample when only reducing to wustite (RedWUS).9)
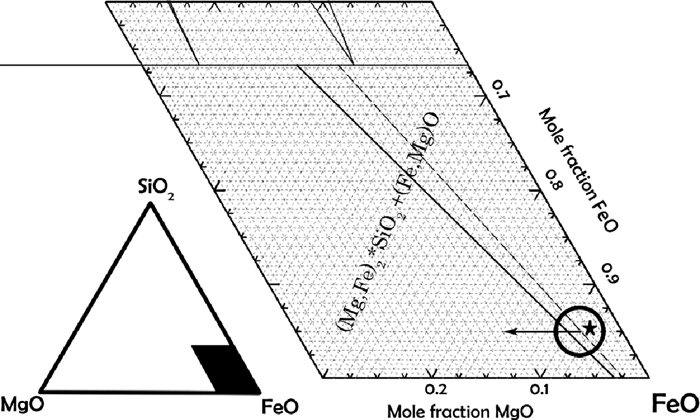
Phase diagram with equilibrium composition for the slag and wustite in the sample reduced at 1100°C. The slag and wustite compositions and tie-line for the sample reduced only to wustite (RedWUS)9) is also superimposed in dashed gray for comparison. The asterisk shows the calculated average composition for the fine olivine samples (S. 2 and S. 4), and the circle the composition determined by the tie-lines in RedWUS.
The difference in magnesium concentrations between the samples reduced to metal and wustite is largest at 1200°C, therefore, the compositions for the slag and the wustite in both these samples were plotted in the phase diagram below.
Comparing the data points in Fig. 13, it is clear that the slag of the metallized samples has been enriched in magnesium compared to those reduced only to wustite at 1200°C. This means that the overall composition is also higher for the metallized sample. A detailed description of the mineralogical behaviour for a molten slag, and an estimation of the overall composition for the fine olivine samples reduced to metal at 1200°C, is offered under the discussion heading.

Phase diagram with equilibrium composition for the slag and wustite, marked by small black asterisks, in the sample reduced at 1200°C. The slag and wustite compositions for the sample reduced only to wustite (RedWUS)9) is also superimposed in pale gray asterisks for comparison. The large asterisk shows the calculated average composition for the fine olivine samples (S. 2 and S. 4), and the circle the composition determined by the tie-lines in RedWUS. A = wustite composition in three-phase region ABC, B = slag composition in three-phase region ABC, C = fayalite composition in three-phase region ABC. D = fayalite composition when the final melt with composition B crystallizes.
The samples reduced at 1300°C show three different compositional cases: the edge, the reduction front and the core, which provide important information on the magnesium diffusion behaviour during cooling of molten phases after reduction above the melting temperature of the slag. Two cases are discussed:
Case 1In S. 1, S. 3 and S. 4 a dense metallic shell formed on the pellet surface, which stopped further reduction. The dense slag layer with small inclusions of wustite extending inside the iron layer (see Fig. 9) has a different magnesium ratio compared to the main pellet body of larger wustite spheres embedded in a fayalitic slag matrix. The pattern of the fine wustite close to the iron is the same as that typical for crystals precipitating from melts and matches well with the phase diagram assuming that the dense material close to the iron was all liquid slag from which small wustite crystals precipitated during cooling. The best match with the phase diagram was obtained for onset of slag crystallization taking place at 1215°C (see Fig. 14). The compositions of the dense fayalitic slag, the fine wustite precipitates as well as the fayalite slag matrix and the larger wustite spheres in the periphereral portion of the main pellet body are marked with asterisks in the phase diagram below at point D, point E, point X and point A, respectively. Superimposed onto the diagram, the tie-line shows the equilibrium between the wustite and slag at the final crystallization at 1164°C of a slag with composition B.

Phase diagram at 1215°C with average compositions for the slag and wustite, marked by black asterisks, in the sample reduced at 1300°C. The large asterisk shows the calculated average composition for the fine olivine samples (S. 2 and S. 4), and the circle the composition determined by the tie-lines in RedWUS.9) A = wustite composition in three-phase region ABC, B = slag composition in three-phase region ABC, C = fayalite composition in three-phase region ABC. D = fayalite composition when the dense fayalitic slag with composition B crystallizes, E = wustite precipitates composition when the final melt with composition B crystallizes and X = fayalite slag matrix composition when the final melt with composition B crystallizes.
Sample 2 (S. 2) reduced at 1300°C reduced almost completely to iron in a similar manner as the pellet with only 0.5% bentonite added. In two of three pellets in S. 2, the metallization was complete, but the the third pellet had a yet unreduced core with MgO-levels of 10% recorded in front of the iron layer. The elevated magnesium levels decayed over a distance of about 500 μm toward the centre before settling at the same level as that of the pellet core in S. 4.
It is very clear that the presence of MgO and SiO2 has an impact on the formation of iron and the physical texture of the iron oxide and iron that forms during the reduction process. However, it also appears that the effect of a certain additive varies between the periphery and the core of the pellets, and also with the temperature. This indicates that the effect of the additives, in this respect, is quite sensitive to temperature and reduction potential (the concentration of the CO-gas decreases and is lower toward the centre). On the macro level it is clear that a higher magnesium to silica ratio influences both the reducibility and the swelling, and especially at 1300°C the impact is very pronounced. Upon reduction only to wustite (RedWUS.),9) the magnesium in the wustite appeared to create a more porous and irregular structure, more open and accessible for reducing gases. The metallic iron formed in the metallized fine olivine samples is also more irregular, with defects and pores, making the total conversion to iron greater in each particle. This effect is especially pronounced in S. 2 reduced at 1300°C, where the iron formed is very porous, with a very “broken up” type of structure with few remaining wustite inclusions. The same structure is also seen in S. 5, but in all the other samples the metal formed in the beginning, probably due to an early presence of liquid slag, has sintered extensively to form a shell closing the structure for further reduction. As the reference (S. 5) reduces very well both at 1200 and 1300°C it appears that the liquid slag components are the ones that cause the sintering and hinder reduction at higher temperatures. It also appears that the high magnesium levels in the slag of S. 2 are able to neutralize the effect of the silica in the sample.
4.2. SwellingThe swelling is greatest for S. 5, and it is the highest at 1000°C, decreasing more as the temperature increases. It appears that the samples with the most binding silica swell the least at 1000°C. The amount of binding media is highest in the order S. 4, S. 2, S. 3, S. 1 and S. 5, as primarily the dissolved olivine, but also the slightly sintered quartzite particles, have bound to surrounding iron oxide particles during oxidation. The higher swelling at 1100°C might be due to the swelling tension being higher due to faster reduction kinetics, or that the silica phase is now fully or partly molten to provide less binding strength. But the samples still keep the relative order of swelling as at 1000°C, which indicates that the silica still plays a role here. At 1200°C the samples swell slightly less, and at 1300°C there is even a net shrinking for S. 3 and S. 4. Now the swelling appears to depend mainly on the amount of quartzite in the sample. It appears that the quartzite facilitates sintering, which becomes important at 1200°C, and becomes the dominant factor at 1300°C. The important factor for swelling between 1000–1100°C therefore seems to be the amount of molten or sintered silica formed during induration, which facilitates binding forces. From 1200°C and above the total amount of silica is the dominant factor, facilitating sintering and contraction of the structure.
4.3. Magnesium DistributionInitially, the question at start of this study was whether the growing metal shell in the pellet during reduction would greatly raise the magnesium levels in the wustite and slag components in the inner part of the pellet, to significantly change the melting conditions for this wustite and slag. The result showed that for the conditions of this reduction study a significant MgO raise in the unreduced wustite occurred only in one case, at 1300°C. In all other cases high MgO-levels were found only in inclusions left behind the reduction front. This was due to relatively long and widespread reduction fronts with gradually more and more iron, which led to only moderate increases in magnesium content.
4.4. 1000–1100°CAt 1000 the presence of the iron front appears to hinder the diffusion from the olivine/magnesiumferrite slightly, so that the level in the slag in the centre of the fine olivine samples is lower compared to that of redWUS.9) In redWUS the magnesium levels had reached equilibrium already at 1000°C, but in the current study there is a distinct increase in magnesium when increasing the temperature from 1000 to 1100°C. This suggests of that the iron might act as a barrier, hindering diffusion of MgO from the olivine particles, especially in the real blast furnace process where most wustite reduces in the indirect reduction zone at 800–1000°C. Since the cores of large olivine particles remain unreactive until above 1100°C, a large part of the olivine magnesium will be entrapped by metal to play a role in the reduction only after melting of the pellet. The reason for the lower level of Mg in S. 4 compared to S. 2 is probably that in S. 2 all slag points chosen for analysis are previous olivine particles, whereas the points in S. 4 might also have been previous quartzite particles. In all samples reduced at 1000–1100°C there is a gradual decrease in metallic iron from the edge until the centre and at no location is the Mg-level significantly different from the levels in the pellet centres presented in Figs. 10 and 11. The MgO level in the centres of the pellets is about 5% compared to 4% in RedWUS,9) (see Fig. 12) which corresponds to a rise in slag melting point by ~15°C(slow temperature rise) according to calculated phase diagrams.
4.5. 1200°CAt 1200°C the presence of the iron front has led to increased magnesium content, so that the relation between magnesium in the wustite and slag indicates that the slag in the centre of the fine olivine pellets (S. 2 and S. 4) was mostly solid, even before onset of cooling. The rise of melting point resulting from this increase in magnesium is ~20°C(slow temperature rise) according to the phase diagram. Hereafter follows an explanation of how this interpretation of the data was done based on the phase diagram in Fig. 13, first for the samples reduced to wustite, then for the metallized samples. The encircled sample average composition for S. 2 and S. 4 in Fig. 13, was determined by the intersection point for the solid-state equilibrium tie-lines between fayalite and wustite at three different temperatures after reduction to wustite. This point lies quite close to the large asterisk, which is the average composition calculated based on the raw material specification. At 1200°C the point for the total composition coincides with line AB, which implies that the equilibrium is between two phases; wustite with composition A and molten slag with composition B. The magnesium of the wustite was measured in the centre of large particles with the assumption that the magnesium level has not changed significantly during the rapid cooling. Upon fast cooling, the liquid slag crystallizes during a temperature interval of 42°C, initially to form fayalite with composition C and new wustite with composition A, and with final crystallization at 1158°C to form fayalite with compostion D and wustite with composition E, according to the superimposed dashed tie-line to the right. The assumption for the solidifying slag with small crystals forming in a liquid is that the crystals will adapt to the changing equilibrium until the final crystallization occurs, so that all fayalite adopts the composition of point D. The new secondary wustite precipitates on the old one, and the two cannot be distinguished. Therefore no measurements could be made on the secondary wustite. The measured Mg-level of the slag, marked with a grey asterix is slightly higher in magnesium than suggested by this explanation. This is assumed to be caused by the flow of magnesium from the wustite particles trying to adapt to the changing equilibrium during cooling. This is supported by results in RedWUS,9) showing that the magnesium content in very small wustite particles were only 0.68–0.84 times the amount in large particles, as they adapted faster to the changing equilibrium during cooling.
At reduction to metallic iron the transformation of wustite to iron concentrates the magnesium in the remaining wustite and slag giving a slag much richer in magnesium (composition labelled with a small black asterisk in Fig. 13). The only way to explain this new slag composition is that the overall composition has drifted left along the horizontal arrow to lie quite close to the tie-line AC, see Fig. 13. That means that most of the slag was solid before cooling with composition C. The presence of a smaller amount liquid of composition C in the slag leads to the slag settling at an overall composition at the point marked with an asterisk in the diagram, when the sample is cooled.
4.6. 1300°CAt 1300°C the composition of the pellet seems to be crucial for the reduction behaviour. The reference(S. 5) and S. 2 with its high Mg to Si ratio have maintained a porous structure whereas S. 1, S. 3 and S. 4 have sintered heavily, so that further access to reducing gases has been cut off. Two cases from this temperature were described under the results heading; firstly, the slag layer inside the metal shell in S. 1, S. 3 and S. 4, and secondly the metal front with very high levels of magnesium in the wustite before the metal in S. 2. The former is discussed in detail, since it proves the cooling mechanism presented for the magnesium in the samples with molten slag.
Case 1In the pellets in S. 1, S. 3 and S. 4 a dense zone of slag with small wustite particles was found between the metal shell and the regular structure of larger wustite spheres embedded in a slag matrix. The composition of the wustite and slag in the dense zone matches very well with the phase diagram if assuming that the wustite and slag in the dense zone were all molten before cooling. The following explanation is offered: After melting of the slag phase and sintering of the wustite to form spheres, the interparticular forces in the liquid makes the wustite spheres pull closer to each other, so that molten slag is pushed out to the periphery to accumulate next to the metal shell. The magnesium in the wustite spheres and surrounding slag matrix matches well with the phase diagram of 1215°C, which indicates that the crystallization of the slag has started at 1215°C during cooling, according to the same mechanism as described for the 1200°C samples. But the 1300°C samples also clearly show the solidification behavior of a slag that crystallizes into new wustite and slag. That was not the case in the 1200°C samples, where the new secondary wustite precipitated on the old wustite, giving no opportunity to separate the two. Also, the flow of magnesium from the wustite to the slag enriched the slag in magnesium, as compared to the point suggested by the mechanism. This is why the composition for the slag matrix in Fig. 14 has drifted left to point x from the theoretical point D suggested by the cooling mechanism. In the periphery of the 1300°C samples, with no wustite spheres in close proximity, the dense slag separates into secondary wustite and fayalite which are almost perfectly aligned with the dashed tie-line for final crystallization in the diagram, see Fig. 14.
Case 2It is clear from the results that the mechanism with an accumulating wave of magnesium moving before a front of advancing metal can lead to Mg-concentrations of much higher orders than when the magnesium is evenly distributed in the pellets. The rise in melting point resulting from the level of 10% MgO recorded just before the reduction front corresponds to 120°C, compared with pure fayalite. The implication of a higher MgO-level would certainly be a longer time period before the silicate portion melts, which would give a greater conversion of wustite to iron and a faster accumulation of Mg in the remaining wustite and slag, which again increases Mg further. When approaching the melting point of the slag, increased sintering reduces reduction rate, which lets the magnesium level decay, thereby reducing the melting point further, which in turn reduces the reduction rate. The magnesium concentration in front of the iron front is hence increased by the reduction, which in in turn appears to increase with an increased Mg-level. The whole process is then a kinetic competition with the reduction rate and the dissolved Mg acting on the positive side, and the sintering of the pellet on the other side. But it is difficult to say if the superior reduction has come mainly as a result of the high magnesium levels or if it was the superior reduction that gave rise to the magnesium accumulation in the first place.
In this study the impact of the growing metal front during reduction of pellets at 1000–1300°C was studied. The following conclusions could be drawn:
Pellets with fine reactive olivine end up with much more magnesium dissolved in the wustite and slag than pellets with coarse unreactive olivine. This is true especially in the temperature range 1000–1100°C, as the unreactive olivine released the main part of its magnesium at 1200°C. But even at 1200°C the magnesium concentration was higher when using fine reactive olivine. The dissolved olivine makes the slag of the pellet separate into fayalitic olivine and an alkali-rich remnant. The swelling of the samples appeared to decrease the higher the amount of fayalitic slag in the sample.
The Mg-concentrations in the metallized samples rise only moderately compared with samples reduced only to wustite if the metal formation occurs without a sharp metal front. However, high Mg-levels result in wustite inclusions entrapped in metallized particles close to olivine particles (up to 80% MgO) and also in inclusions behind the metal front (up to 50% MgO).
Higher levels of MgO in the yet non-metallized portion of the pellets occur only in case of iron formation with a sharp metal front. This was the case at 1300°C for the sample with addition of only fine olivine, where the MgO content just before the reduction front measured ~10% MgO. At equilibrium with a fayalite slag this MgO content implies a rise of melting point of the slag by 120°C, according to thermodynamic calculations. The high MgO/SiO2 resulting from the addition of fine olivine significantly improved the reducibility at this temperature, where formation of a dense metal shell hindered reduction in most other samples.
Financing from HLRC (Hjalmar Lundbohm Research Centre) and CAMM (Centre for Advanced Mining and Metallurgy) is gratefully acknowledged. We would also like to thank LKAB for providing material, as well as valuable assistance and discussions about tests and results.