1. Introduction
Because of the scarcity of high grade hematite ores, it is necessary to use magnetite ores in the blast furnace ironmaking or DR processes.1) The gas-based DR processes, such as, MIDREX or HYL III is becoming more comparative due to low price of shale gas. Nevertheless, coal-based DRI processes, such as, rotary kiln or rotary hearth furnace are still receiving great attention for producing flat product of steel in terms of a virgin iron.2) Extensive understanding of DRI production technology using low grade ores is limited in alternative ironmaking processes. Great concerns have been taken about the reduction of titanomagnetite (TTM) ore, so-called iron sand since it is not easily reducible.3)
The basic researches about the mechanism and kinetics of carbothermic reduction of iron oxides were extensively carried out. Rao4) found that the availability of CO governs the reduction process in the mixture of hematite and graphite and that the carbon gasification reaction determines the rate controlling step for the overall process. Srinivasan and Lahiri5) showed that the activation energy of the carbothermic reduction of hematite by graphite has been found to decrease with progress in reduction, indicating a possible changeover in reaction mechanism. Fruehan6) confirmed that the reduction of Fe2O3 to FeO takes place by means of the gaseous intermediates CO and CO2, and that the overall rate is controlled by the carbon oxidation by CO2. Recently, Yang et al.7) reported that the direct reduction at initial stage dominates carbothermic reduction of hematite-graphite composite and that it is determined by the indirect reduction together with the carbon gasification reaction. Chen et al.8) showed that the metallization degree of TTM concentrates are significantly improved by the pre-oxidation and by adding additives in the solid state reduction of TTM ores with pulverized coal.
Most of the reduction degrees in the carbothermic reduction were evaluated by the weight change using TGA for the stoichiometric mixture of iron oxide and graphite. However, the continuous change of reduction degree of raw iron ore and char composite was not clearly evaluated in terms of the weight change by TGA. In the current study, it was undertaken to obviously evaluate the fractional reduction of raw iron ore and char composite in terms of TGA data. The fractional reduction evaluated by TGA was validated by the chemical analysis of the oxygen combined with Fe in the reduced TTM.
2. Theoretical Background
Reducibility of ferrous materials is characterized by their fractional oxygen removal rates in gaseous reducing atmosphere. The fractional oxygen removal is defined by Eq. (1):9)
where
n0 is the number of moles of oxygen originally combined with Fe only and
n is the number of moles of oxygen left combined with Fe after experimental time,
t. However, according to some researches
4,6) about the carbothermic reduction of Fe
2O
3 with graphite, the fractional reduction was evaluated for the reduction of Fe
2O
3 and graphite composite as follows:
where
Wo is the initial pellet weight before reduction (g),
Wt is the pellet weight decrease (g) during time,
t. In
Eq. (2),
M represents the fraction of weight loss corresponding to complete reduction of Fe
2O
3. Based on the fractional reduction, the uniform internal reduction model was proposed for the carbothermic reduction of iron oxide and graphite composite under the assumption that the overall reaction is determined by carbon gasification reaction.
4,6)
where
k represents the rate constant of the carbothermic reduction reaction. As a pseudokinetic parameter, the fractional reduction (
f) has been used by the previous researchers to measure the reduction rates although it can only provide an apparent idea about the extent and characteristics in the progress of reduction.
10,11)
Yang et al.7) evaluated the reduction degree for the samples withdrawn in the progress of the reduction in terms of the weight change of the pellet. With some assumption that the total amount of reduction of iron oxides by CO is equal to the amount of the carbon gasification reaction, the overall reduction can be expressed as follows:
|
Fe
2
O
3
(
s
)
+3C(
s
)
=2Fe(
s
)
+3CO(g)
| (4) |
They used the similar definition of the fractional reduction to that by Rao.4) In Eq. (2), M corresponds to 0.4293 for the case of Fe2O3 since M is equal to 3MWCO/(MWFe2O3 + 3MWC) in case the Reaction (4) goes to completion.
Takeuchi et al.12) estimated the fractional reduction of the wustite sample by Eq. (4) although it was a gaseous reduction:
|
f=
(O/Fe)
0
-(O/Fe)
(O/Fe)
0
| (5) |
where (O/Fe)
0 and (O/Fe) are the ratios between oxygen combined with Fe in mass% and total Fe in mass% in the samples before and after reduction.
Equation (5) can mostly be used to exactly estimate the fractional reduction of any iron ore or oxide by gaseous reduction based on the results of chemical analyses. However,
Eq. (5) cannot be applied to continuously monitor the change of the fractional reduction of iron ores or oxides.
3. Experimental
3.1. Materials
Table 1 shows the chemical compositions of titanomagnetite mined in New Zealand. The average size of ore particles is about 190 μm. It is well known that the titanomagnetite ore in New Zealand is a solid solution of magnetite and ulvospinel as expressed by Eq. (6):3)
|
(
Fe
3
O
4
)
1-x
(
Fe
2
TiO
4
)
x
⇒
(
FeO⋅
Fe
2
O
3
)
1-x
(
Fe
2
TiO
4
)
x
| (6) |
Table 1. Chemical compositions of raw titanomagnetite used in the present study (mass%).
| Total Fe | FeO | Fe2O3 | SiO2 | Al2O3 | TiO2 | MgO | CaO | MnO |
|---|
| 57.79 | 28.65 | 50.78 | 3.31 | 3.67 | 7.00 | 3.32 | 1.63 | 0.45 |
Based on the compositions in Table 1, the mole fraction of ulvospinel in titanomagnetite, x was evaluated to be 0.28 using Eqs. (7) and (8), which is in good agreement with 0.27 ± 00.02 previously estimated by microscopic line mapping.3)
|
Mole
of
FeO
:
mole
of
TiO
2
=
(
1
+
x
)
:
x
| (7) |
|
mass%FeO
M
W
FeO
:
mass%TiO
2
M
W
TiO
2
=(
1+x
)
:x
| (8) |
The char used in the current study was prepared by heating a coal at 1123 K for 3 hours in N2 atmosphere and it was analyzed to contain 2.63 mass% of volatile matter (VM) and 10.1 mass% of ash.
3.2. Experimental Procedure
Pulverized titanomagnetite (about 81 μm) and char (about 10 μm) were homogeneously mixed with a stoichiometric ratio using a ball mill. The pellets were formed into cylindrical shape (10 mm diameter and 7 mm height) weighing 1.5 g (±0.01 g) by a hydraulic press under the pressure of 50 MPa for 1 min with distilled water added to ensure complete compactness. The pellets were air dried in an oven at 423 K before use. The pellet was placed into a cylindrical Al2O3 crucible (diameter 14 mm, height 20 mm) which was then suspended and connected using a Pt wire to the balance part of a thermogravimetric analysis (TGA) apparatus (RUBOTHERM, Germany). The isothermal reduction experiments were performed rapidly by heating up the TGA furnace at 100 K/min to 1473 K. The flow rate of Ar carrier gas was preliminarily determined to be 250 mL/min.
4. Results and Discussion
4.1. Evaluation of Fractional Reduction of Tatanomagnetite (TTM) and Char Composite by TGA
The raw TTM was analyzed by chemical analyses as shown in Table 1. From the weight percent of FeO and Fe2O3, the oxygen content combined with Fe was calculated to be about 21.64 mass%, which is the theoretical loss in weight per 100 g of TTM for complete reduction by Eqs. (9) and (10).
|
O in FeO=(
mass%Fe
2+
)
×
M
W
O
M
W
Fe
=6.38 mass%
| (9) |
|
O in Fe
2
O
3
=(
mass%Fe
3+
)
×
3M
W
O
2M
W
Fe
=15.26 mass%
| (10) |
In order to calculate the fractional reduction of TTM and char composite, it is necessary to estimate the stoichiometric amount of char required to reduce all the oxygen combined with Fe in the TTM in such a way that the molar ratio of carbon to oxygen (C/O = 1) is unity based on the following reduction reaction considering that the TTM contains 0.2164 g in 1 g of TTM and that the fixed carbon of the char is 87.32 mass% in such a way that the molar ratio of carbon to oxygen (C/O) is unity:
|
(
Fe
3
O
4
)
1-x
(
Fe
2
TiO
4
)
x
+char=reduced TTM+CO
| (11) |
|
Amount of required char(
g
)
=0.2164 g×
M
W
C
M
W
O
×
100
87.32
=0.1859 g
| (12) |
where
MWi represent the molecular weight of component
i. This results in the estimation of char amount to be 0.1859 g to reduce 1 g of TTM. The weight change by TGA consists of the weight change of TTM and that of char according to the reduction by
Eq. (11). That is, one oxygen atom in TTM is removed together with one carbon atom with the progress of reduction. Therefore, the weight change of TTM can be calculated by
Eq. (15):
|
Δ
W
TGA
=Δ
W
TTM
+Δ
W
char
| (13) |
|
Δ
W
TTM
:Δ
W
char
=16:12
| (14) |
|
Δ
W
TTM
=Δ
W
TGA
×(16/28)
| (15) |
The fractional reduction of TTM can be evaluated by the weight change of TTM with respect to the oxygen content combined with Fe in TTM as represented by
Eq. (16).
|
Fractional reduction=
Δ
W
TTM
W
TTM,i
×0.2164
| (16) |
|
W
TTM,i
=
W
(
TTM+char
)
, i
×
1
1+0.1859
| (17) |
|
Fractional reduction=
Δ
W
TGA
×(16/28)
W
(
TTM+char
)
,i
×
1
1+0.1859
×0.2164
| (18) |
Combination of
Eqs. (16) and
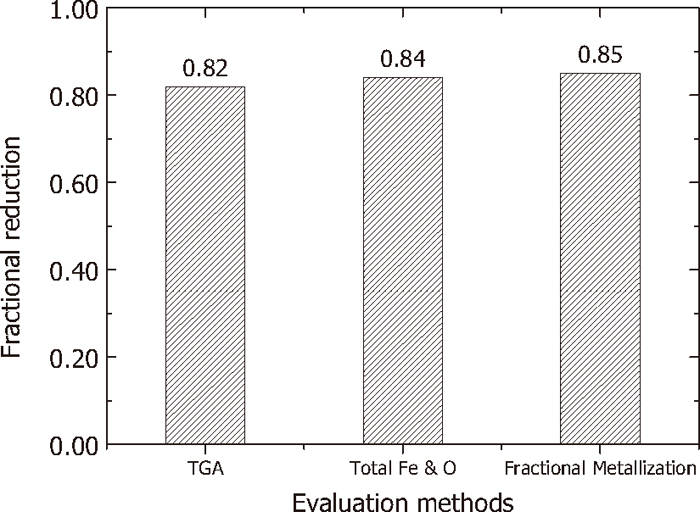
(17) provides the resulting equation for evaluating the fractional reduction of TTM and char composite with the information of the weight change of the pellet by TGA and the initial weight of pellet with the mixing ratio of TTM and char. For example, if the pellet weights before and after the reduction experiment were 1.496 g and 1.104 g, respectively, the fractional reduction can be evaluated to be 0.82 based on the reduction experiment for which composition of reduced TTM is shown in
Table 2.
Table 2. Chemical compositions of titanomagnetite and char composite after reduction (mass%).
| Total Fe | FeO | Fe2O3 | Metallic Fe | SiO2 | Al2O3 | TiO2 | MgO | CaO | MnO |
|---|
| 66.31 | 9.62 | 3.36 | 56.48 | 4.56 | 4.61 | 8.54 | 3.93 | 1.29 | 0.61 |
The approach employed for evaluating the fractional reduction of TTM by TGA can be validated by applying it to the carbothermic reduction of Fe2O3 by graphite according to Reaction (4).7,13) That is, the fractional reduction of carbothermic reduction of Fe2O3 can be evaluated based on the Reaction (4) considering that 1 g of Fe2O3 contains 0.3006 g of oxygen and the fixed carbon in graphite is 100 mass%:
|
Amount of required graphite(
g
)
=0.3006 g×
3M
W
C
3M
W
O
=0.2255 g
| (19) |
The subsequent procedure is quite similar to that of evaluating the fractional reduction of TTM and char composite using
Eqs. (13),
(14),
(15),
(16),
(17),
(18).
|
Fractional reduction=
Δ
W
Fe
2
O
3
W
Fe
2
O
3
,i
×0.3006
| (20) |
|
W
Fe
2
O
3
,i
=
W
(
Fe
2
O
3
+graphite
)
,i
×
1
1+0.2255
| (21) |
|
Fractional reduction=
Δ
W
TGA
×(16/28)
W
(
Fe
2
O
3
+graphite
)
,i
×
1
1+0.2255
×0.3006
=
Δ
W
TGA
W
(
Fe
2
O
3
+graphite
)
,i
×0.4293
| (22) |
As previously mentioned, 0.4293 corresponds to the value,
M in
Eq. (2) for the carbothermic reduction of Fe
2O
3 by graphite since
M is equal to 3
MWCO/(
MWFe2O3 + 3
MWC) in case the Reaction (4) goes to completion. The similar application to the case for the carbothermic reduction of Fe
3O
4 by graphite would yield 0.4006 as the value of
M because
M corresponds to 4
MWCO/(
MWFe3O4 + 4
MWC).
14) The above results indicate that
Eq. (18) can generally be applied to the evaluation of the fraction reduction of carbothermic reduction of ore by carbon for any type of iron ore just relying on the weight change of ore and carbon composite by TGA.
4.2. Calculation of Final Fractional Reduction by Total Fe and O Combined with Fe in Reduced TTM
As previously explained, the fraction reduction estimated by TGA can be justified by analyzing total Fe and oxygen content combined with Fe of the reduced TTM as follows:
|
O in FeO=(
mass%Fe
2+
)
×
M
W
O
M
W
Fe
=2.98 mass%
| (23) |
|
O in
Fe
2
O
3
=(
mass%Fe
3+
)
×
3M
W
O
2M
W
Fe
=1.09 mass%
| (24) |
From the weight percent of FeO and Fe
2O
3 in
Table 2, the oxygen content combined with Fe was calculated to be about 4.08 mass%, which was used to calculate the final fractional reduction of 0.84 by
Eq. (5). Considering the uncertainty in the chemical analysis of total Fe and ferrous ion in the reduced TTM, this confirms that the calculated fractional reduction value is almost similar to that evaluated by
Eq. (18) as shown in
Fig. 1. This indicates that the fractional reduction of carbothermic reduction of raw iron ore can simply be evaluated by TGA data only, which enables to calculate the apparent rate constant in uniform internal reduction model in the progress of iron ore and carbon composite by
Eq. (3).
4.3. Comparison of Final Fractional Reduction with Fractional Metallization Based on Chemical Analysis
In actual production process of direct reduced iron (DRI), the final reduction degree of DRI has been evaluated in terms of metallization degree by analyzing the concentrations of total Fe and metallic Fe in the reduced ore. Chen et al.8) evaluated the progress of carbothermic reduction of titanomagnetite concentrates in terms of fractional metallization degree by Eq. (25):
|
η=
(mass%M.Fe)
(mass%T.Fe)
| (25) |
where mass%T.Fe and mass%M.Fe represent mass percents of total Fe and metallic Fe in the reduced sample, respectively.
Equation (25) can be used to estimate the reduction progress of carbothermic reduction by chemical analysis right after the formation of metallic Fe in the sample withdrawn out of the reduction chamber. However, it is not possible to understand the reduction behavior among iron oxides in the initial stage of reduction before the formation of metallic Fe. In the experimental data of reduction used in the previous section, the fractional metallization was calculated to be 0.85, which is close to that obtained by chemical analysis of total Fe and O content combined with Fe. Therefore, the fractional reduction evaluated by TGA data can sufficiently be utilized for monitoring the progress of carbothermic reduction of raw iron ores by confirming its validity in terms of fractional reduction and fractional metallization degree by chemical analyses.
5. Conclusions
It was undertaken to continuously estimate the fractional reduction of raw titanomagnetite and char composite by using TGA data with the intention of evaluating the rate constant in the uniform internal reduction model. The approach method of evaluating the fractional reduction of TTM by TGA was validated by applying it to the carbothermic reduction of Fe2O3 and Fe3O4. For further generalization of the attempted evaluation, the fraction reduction estimated by TGA was justified by analyzing total Fe and oxygen content combined with Fe in the reduced TTM. In addition, the fractional reduction of raw TTM and char composite was compared with the fractional metallization widely used in actual production process of direct reduced iron.
References
- 1) S. Inaba: Tetsu-to-Hagané, 87 (2001), 221.
- 2) F. Globler and R. C. A. Minnitt: J. South African Inst. Min. Metall., (1999), March/April, 111.
- 3) E. Park and O. Ostrovski: ISIJ Int., 43 (2003), 1316.
- 4) Y. K. Rao: Metall. Trans., 2 (1971), 1439.
- 5) N. S. Srinivasan and A. K. Lahiri: Metall. Trans. B, 8B (1977), 175.
- 6) R. J. Fruehan: Metall. Trans. B, 8B (1977), 279.
- 7) J. Yang, T. Mori and M. Kuwabara: ISIJ Int., 47 (2007), 1394.
- 8) D. Chen, B. Song, L. Wang, T. Qi, Y. Wang and W. Wang: Miner. Eng., 24 (2011), 864.
- 9) A. K. Viswas: Principles of Blast Furnace Ironmaking, SBA Pub., Calcutta, India, (1981), 216.
- 10) S. B. Sarkar and H. S. Ray: Trans. Iron Steel lnst. Jpn., 28 (1988), 1006.
- 11) S. B. Sarkar, H. S. Rayand and I. Chatterjee: J. Thermal. Anal., 35 (1989), 2461.
- 12) N. Takeuchi, Y. Nomura, K. Ohno, T. Maeda, K. Nishioka and M. Shimizu: ISIJ Int., 47 (2007), 386.
- 13) S.-M. Jung and S. H. Yi: Steel Res. Int., 84 (2013), in press.
- 14) S.-M. Jung and S. H. Yi: Ironmaking Steelmaking, 40 (2013), in press.