Abstract
To achieve the action mechanism and optimal usage of dolomite in the sintering process, solid reaction tests, fluidity tests, bonding strength tests and sinter pot tests were conducted, then microstructure and energy spectrum were analysed further. Reaction temperature of dolomite with calcium ferrite was lower than that with limonite iron ore about 155°C. Fluidity of bonding phase and strength of sinter using dolomite were lower than that using light burned dolomite and serpentine owing to the higher MgO content in the calcium ferrite and formation of secondary bonding phase. Action mechanism of dolomite in sintering process mainly included 4 steps, thermal decomposition, solid reaction, formation of primary bonding phase and formation of secondary bonding phase. Dolomite mainly played it’s role in the third step in which high viscosity CFM (calcium ferrite with MgO) and magnetite solid solution formed. Increasing the solid solubility of MgO in magnetite by mixing dolomite with magnetite concentrate before granulation, decreasing the dispersity of MgO in sinter mixture by increasing the particle size of dolomite appropriately, increasing the separation degree of MgO and CaO by light-burning the dolomite, and decreasing the reaction amount of MgO by using big particle size magnesia, all could decrease the content of MgO in calcium ferrite, thus showed good performance in sinter pot tests.
1. Introduction
With the increase of iron ore consumption in iron and steel industry all over the world, obvious degradation of high quality iron ores occurs,1,2) some low grade and high SiO2 iron ores are also newly developed,3) which leads to increase of SiO2 content in the sinter mixture, and then increase of slag content of sinter. In order to keep the grade and slag content of sinter, consumption of serpentine containing SiO2 and MgO has to be decreased or stoped, dolomite containing CaO and MgO or other MgO-bearing materials with few SiO2 content are used as substitution of serpentine and limestone.
Many investigations on differences between dolomite and serpentine use in sintering process have been reported. Kasai4,5) found that on account of the low proportion of MgO-bearing materials in mixture, little differences on thermal decomposition of mixture between using fine dolomite and fine serpentine, especially above 1200°C, but on the case of same CaO concentration, compared to serpentine, mixtures using dolomite had higher melting down temperature. Panirahy and Qian6,7,8) found that drum index and yield of product were decreased by adding dolomite in sinter to increase MgO content, while by adding serpentine, the drum index and yield were increased. Higuchi9) found that formation of magnetite by reaction between MgO and calcium ferrite melt was associated with the decrease in strength or yield of sinter. H. Li10) found that increase in MgO content, introduced by addition of dolomite, decrease the amount of calciun ferrite slightly, but when MgO was added in the form of serpentine the calcium content increased considerably. In addition, influence of MgO content on the softening property and reducibility of sinter were also researched.11) However investigations focusing on action mechanism of dolomite, such as the distribution of MgO in magnesia solid solution and calciun ferrite, as well as it’s influence on the fluidity and bonding capacity of bonding phase, then the further reaction between primary bonding phase and the nuclei ore in the iron ore sintering process are limited. In addition, the research of optimal usage of dolomite are also needed to be explored.
Therefore, in the present research, firstly, action mechanism of dolomite in the sintering process in comparison with that of light burned dolomite and serpentine was discussed on the base of some fundamental tests, such as solid reaction temperature tests, fluidity of liquid phase tests and bonding strength tests. Based on these results, sinter pot tests were performed to achieve the optimal usage of dolomite in sintering process.
2. Experiment
2.1. Raw Materials
Dolomite, l-dolomite (Light burned dolomite), serpentine, magnesia, quick lime, limestone and iron ore fines were used in this research. All the fluxes were produced in China, Ore-A was limonite ore from Australia, Ore-B was hematite ore from Brazil, Ore-C was blending ore from a Chinese steel mill. Table 1 shows the chemical composition of the fluxes and ores used in this study. Table 2 shows particle size of dolomite comparing with other fluxes in the sinter pot tests. In addition, 3 different particle size distribution dolomite were used.
Table 1. Chemical composition of fluxes and ores used in this study (mass%).
| T.Fe | SiO2 | CaO | MgO | Al2O3 | LOI |
|---|
| Dolomite | 0 | 1.88 | 31.12 | 19.64 | 0.44 | 45.73 |
| L-Dolomite | 0 | 1.37 | 57.69 | 36.69 | 0 | 4.00 |
| Serpentine | 0 | 38.19 | 2.13 | 38.05 | 0.93 | 12.07 |
| Magnesia | 0 | 1.37 | 1.40 | 97.00 | 0 | 0.30 |
| Quick lime | 0 | 0 | 76.23 | 1.00 | 0 | 15.74 |
| Limestone | 0 | 1.08 | 53.59 | 0.63 | 0.40 | 43.59 |
| Ore-A | 58.07 | 5.30 | 0.03 | 0.05 | 1.55 | 10.18 |
| Ore-B | 65.26 | 1.92 | 0.03 | 0.03 | 1.27 | 2.08 |
| Ore-C | 60.33 | 4.30 | 0.32 | 0.07 | 1.50 | 5.58 |
Table 2. Particle size distribution of dolomite and other fluxes in pot tests (mass%).
| 10–12 mm | 6.3–10 mm | 3.15–6.3 mm | 1–3.15 mm | 0.5–1 mm | 0.25–0.5 mm | –0.25 mm | Mean Size |
|---|
| Dolomite-1 | 0 | 0 | 0 | 2.99 | 7.25 | 15.69 | 74.07 | 0.27 |
| Dolomite-2 | 0 | 0 | 0 | 39.30 | 12.30 | 11.20 | 37.20 | 1.00 |
| Dolomite-3 | 0 | 0 | 29.30 | 27.80 | 8.70 | 7.90 | 26.30 | 1.90 |
| L-Dolomite | 0 | 0 | 0 | 4.28 | 5.64 | 20.16 | 69.92 | 0.29 |
| Serpentine | 0 | 0 | 0 | 10.95 | 15.84 | 28.42 | 44.80 | 0.51 |
| Magnesia | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 8.15 |
| Quick lime | 0 | 0 | 0.15 | 5.48 | 6.00 | 12.28 | 76.11 | 0.31 |
| Limestone | 0 | 0 | 0 | 5.24 | 10.10 | 19.74 | 64.93 | 0.34 |
To clarify the reaction temperature of the MgO-bearing materials with iron ore or low melting point calcium ferrite, solid reactivity tests were carried out. Figure 1 shows the schematic diagram of the solid reaction, the reaction temperature when flux reacted with solid ore or CF (calcium ferrite) but no liquid phase formed was defined as the Reaction Temperature (RT). The particle size of fluxes and ore were below 0.15 mm, CF was mixed with Fe2O3 and CaO pure reagent in the constant proportion 74:26 in mass ratio, iron ore was ore-A from Australia. Materials were shaped into tablets with different diameters by using two steel moulds under a pressure of 15 Mpa. The Flux tablet was 2 g of weight with using different MgO-bearing materials, by contrast, the Fe2O3 bearing materials tablet was made by 0.8 g ore or CF. Tablets were sintered in a specific experiment device named Micro-Sinter equipment.12) Figure 2 shows the temperature system and atmosphere of the reaction temperature test.
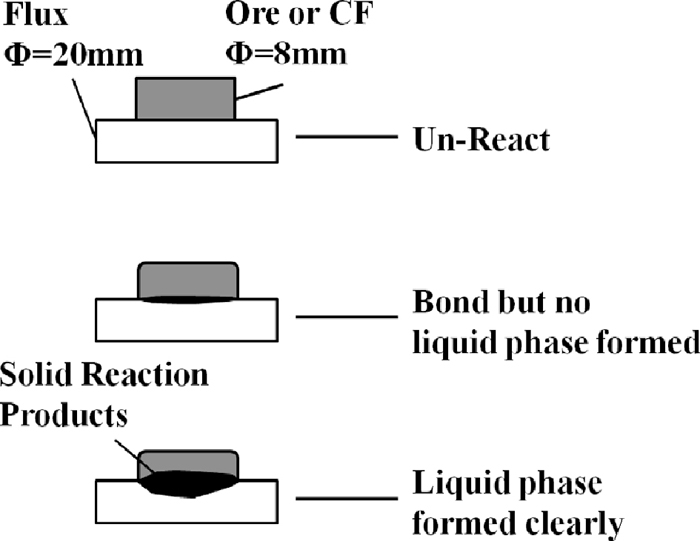
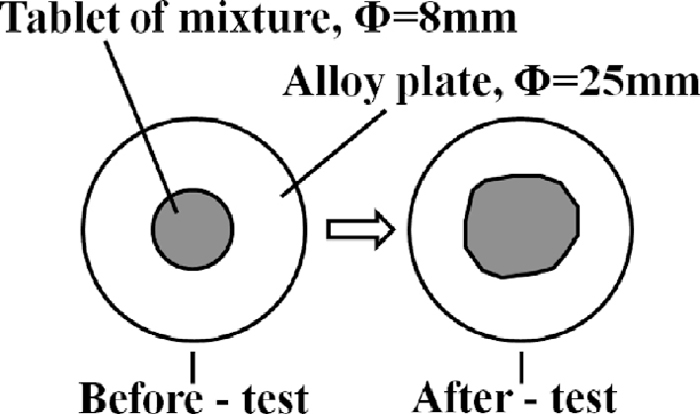
To clarify the influence of the MgO-bearing materials on the Fluidity of liquid phase in the sintering process, fluidity tests were carried out by the Fluidity of liquid phase tests method.13) Dolomite, l-dolomite and serpentine below 0.15 mm were used, Fe2O3, SiO2, CaO, MgO and Al2O3 pure reagent were also used. Table 3 shows the mixing condition and particle size of the materials used in the fluidity tests. To achieve the influence of MgO on the fluidity of liquid phase clearly, MgO and SiO2 content in the fine mixture were higher than normal sintering adhesive powder, and the chemical composition of the fine mixture was maintained constant, i.e. Fe2O3 = 45.8 mass%, SiO2 = 6.95 mass%, CaO = 20.69 mass%, MgO = 5.81 mass%, Al2O3 = 1.06 mass%, basicity was 3.0, by adjusting the proportion of pure reagent. In addition, dolomite with 3 different particle size (–0.15 mm, 0.15–0.25 mm, 0.25–0.50 mm) were used in fluidity tests to reveal the influence of the dispersity of MgO on the fluidity of liquid phase in the sintering process. Fine mixture about 0.8 g was shaped into tablet by using steel mould under a pressure of 15 Mpa. The equipment, temperature and atmosphere of fluidity tests were almost same to the solid reaction tests, expect that the experiment temperature of fluidity tests was 1280°C, and the air atmosphere was changed to N2 after 600°C. Figure 3 shows the schematic diagram of fluidity of liquid phase. Index of fluidity of liquid phase (IFL) was achieved by comparing the area of the liquid phase before and after tests. As shown in Eq. (1), IFLs were calculated.
Table 3. Mixing conditions and particle size of fine mixtures (mass%).
| Size (mm) | Sch. 1 | Sch. 2 | Sch. 3 | Sch. 4 | Sch. 5 |
|---|
| Dolomite | –0.15 | 26.05 | 0 | 0 | 0 | 0 |
| Dolomite | 0.15–0.25 | 0 | 26.05 | 0 | 0 | 0 |
| Dolomite | 0.25–0.50 | 0 | 0 | 26.05 | 0 | 0 |
| L-Dolomite | –0.15 | 0 | 0 | 0 | 15.84 | 0 |
| Serpentine | –0.15 | 0 | 0 | 0 | 0 | 15.00 |
| Fe2O3 | –0.15 | 57.38 | 57.38 | 57.38 | 64.82 | 63.00 |
| CaO | –0.15 | 10.12 | 10.12 | 10.12 | 11.55 | 20.00 |
| SiO2 | –0.15 | 5.63 | 5.63 | 5.63 | 6.73 | 1.10 |
| Al2O3 | –0.15 | 0.82 | 0.82 | 0.82 | 1.06 | 0.90 |
In which, IFL is Index of fluidity of liquid phase, FA is Flowing Area, mm2, and OA is Original Area, mm2.
2.4. Bonding Strength
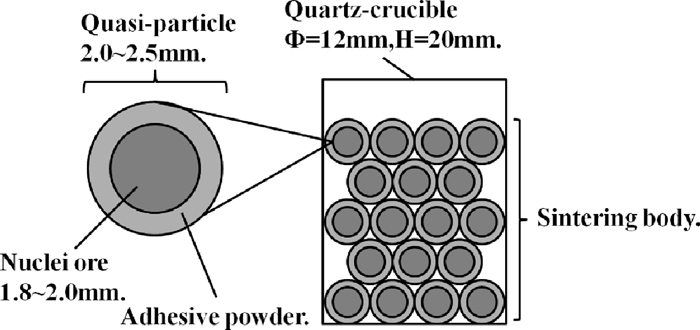
To clarify the influence of MgO in different occurrence states on the strength of sinter, bonding strength tests were carried out. Dolomite, l-dolomite and serpentine below 0.15 mm were used as MgO-bearing materials, and Fe2O3, SiO2, CaO, MgO and Al2O3 pure reagent were also used. Figure 4 shows the schematic diagram of sintering body in bonding strength tests. About 200 quasi-particles made in a disc pelletizer were put into a quartz crucible. The nuclei-particles used in this tests were Ore-B from Brazil, and the adhesive powder were consist of MgO-bearing materials, Ore-C and pure reagent. To achieve the influence of MgO on the bonding strength clearly, MgO and SiO2 content in quasi-particles were higher than normal sinter, the chemical composition of quasi-particles were maintained constant, i.e. Fe2O3 = 53.3 mass%, SiO2 = 5.93 mass%, CaO = 11.86 mass%, MgO = 3.70 mass%, Al2O3 = 1.26 mass%, basicity was 2.0, by adjusting the proportion of pure reagent. The equipment, temperature and atmosphere of bonding strength tests were same to the fluidity tests. After sinter, measured the weight (original weight) of the sinter body, and then free fell the sinter body from 2 m high, and screened the pieces with 5 mm perforated screen, first shatter index was achieved by calculating the ratio of the weight of pieces above 5 mm and the original weight of the sinter body. Then sequentially free fell the above 5 mm pieces from 2 m high and screened, second shatter index was achieved by calculating the ratio of the weight of pieces above 5 mm and the original weight of the sinter body. The third to ten shatter indexes were achieved similarly.
3. Results
Figure 5 shows the results of reaction temperature tests of different MgO-bearing materials. The reaction temperatures of MgO-bearing materials with Ore-A were much higher than that of MgO-bearing materials with CF, for dolomite, the difference was 155°C. In addition, the reaction temperature of dolomite with CF was higher than that of light burned dolomite about 14°C, and lower than that of serpentine about 44°C. It is speculated from these results that in sintering process, MgO-bearing materials rarely reacted with iron ores directly, but reacted with the CF (formed after 500°C by solid reaction between iron ore and CaO-bearing materials, such as quick lime or limestone) by solid reaction. But due to the poor dynamic condition, especially for the big size MgO-bearing materials, solid reaction were limited, so most of the MgO-bearing materials fused into calcium ferrite and reacted with other materials after the melt formation.
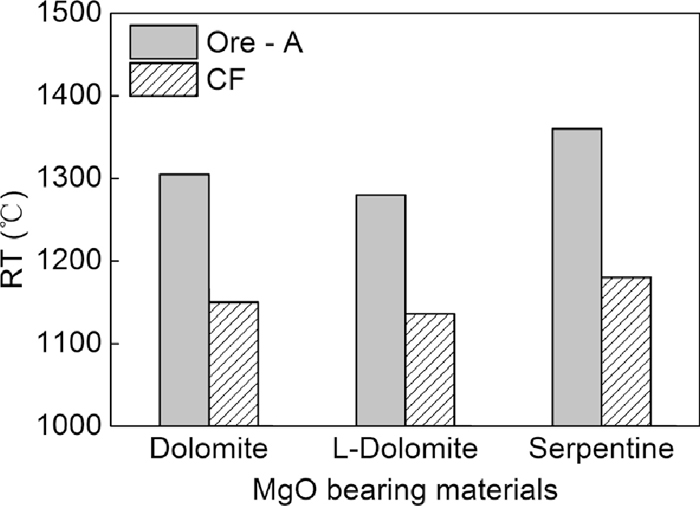
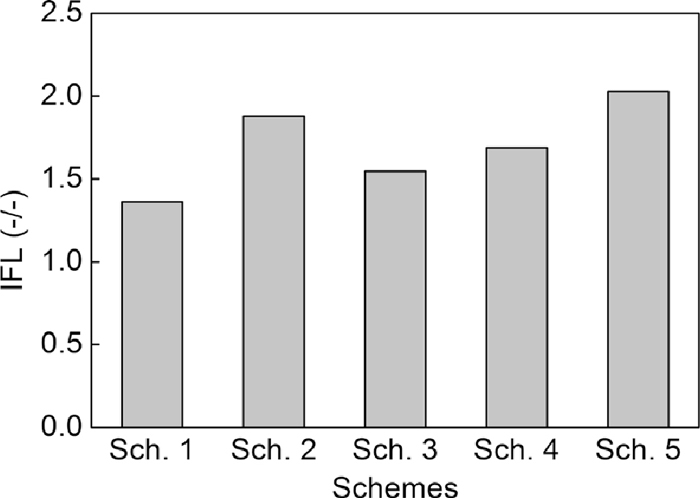
Figure 6 shows the results of fluidity of liquid phase tests. Among Sch. 1 to Sch. 3, Sch. 2 using 0.15–0.25 mm dolomite had the highest IFL, then Sch. 3 using 0.25–0.5 mm dolomite and Sch. 1 using –0.25 mm dolomite. These may be caused by the dispersity of MgO and reaction ratio of CaO in dolomite. In addition, IFLs of Sch. 4 using light-burned dolomite, Sch. 5 using serpentine were all higher than Sch. 1. It is seemed to indicate that compared to dolomite, light-burned dolomite and serpentine had better effect on the fluidity of liquid phase, and certainly, all these results were caused by different action mechanism of MgO-bearing materials which will be analyzed later.
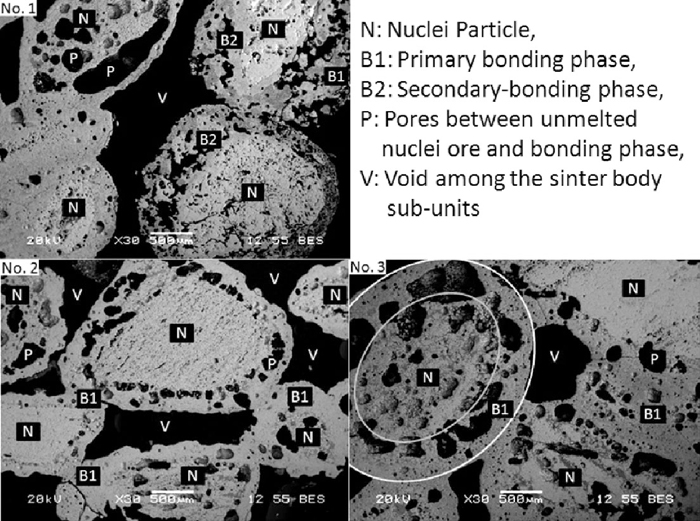
Figure 7 shows the macroscopic feature of the sinter body in the bonding strength tests. Sinter body was composed by a lot of sinter body sub-units, sinter body sub-units of NO. 1 bonded each other deficiently, on the contrary, sinter body sub-units of NO. 3 bonded each other effectively.
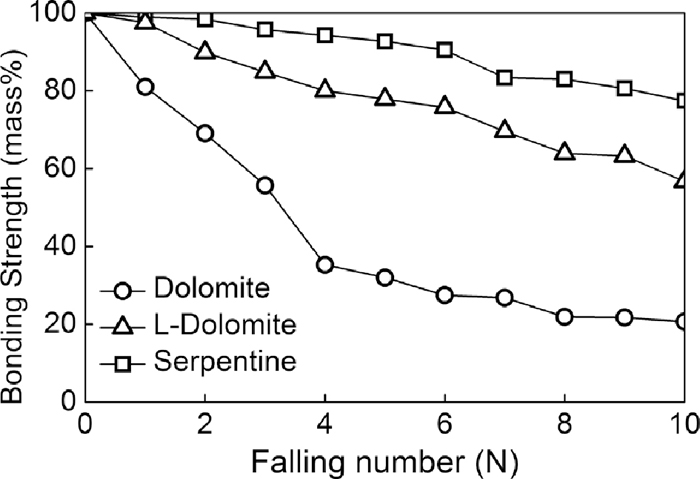
Figure 8 shows the shatter indexes of bonding strength tests. Bonding strength of different schemes was influenced by the occurrence state of MgO. Similar to the fluidity tests results, adhesive power using serpentine had highest bonding strength, then light-burned dolomite and dolomite. These results were seemed to be caused by different fluidity and bonding mechanism of bonding phase which will be analysis later.
Figure 9 shows the relationship between fluidity and the bonding strength. Bonding strength increased with the increase of fluidity of liquid phase. In the sintering process, most of the MgO-bearing materials kept in adhesive powder rather than nuclei ore, so high fluidity of liquid phase supported high bonding capacity of bonding phase, then high strength of sinter.
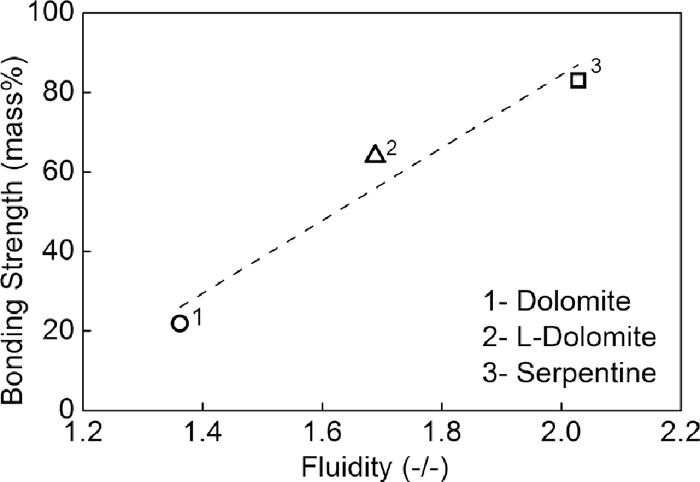
Figure 10 shows the microscopic feature of the sinter body in the sinter strength tests. Sinter body was composed by void and sinter body sub-units as shown in NO. 1 to NO. 3. Each sinter body sub-unit was composed by un-melted nuclei ore, bonding phase and pores as shown by the red circle and yellow circle in NO. 3. Compared to serpentine and light burned dolomite, for the sinter using dolomite, void among sinter body sub-units was bigger. In addition, two kinds of bonding phase were discovered clearly in the sinter body using dolomite. In this paper, the bonding phase formed by the internal reaction in the adhesive powder was named as primary bonding phase (B1), on the contrary, the bonding phase formed by the reaction between primary bonding phase and the nuclei ore was named as secondary bonding phase (B2). With the decrease of the fluidity of primary bonding phase, the contact time and contact area between primary bonding phase and nuclei ore increased, which promoted the reaction between primary bonding phase and the nuclei ore. In addition, the mineral composition of the primary bonding phase could also influence the formation reaction of secondary bonding phase. Certainly, the formation mechanism and influencing factors of secondary bonding phase were also needed to be studied further. It can be speculated from these results that the adhesive powder using dolomite as MgO-bearing material had low fluidity in the sintering process, and could not bond the sinter body sub-units together as also shown in Figs. 6 to 8, moreover, part of the low fluidity bonding phase surrounding the nuclei ore closely reacted with the nuclei ore further, and produced secondary bonding phase which decreased the fluidity of bonding phase again. In contrast, the high fluidity bonding phase using serpentine could bond the sinter body sub-units together effectively and left small void, moreover, the primary bonding phase reacted with the nuclei ore a little and left bigger pores between un-melted nuclei ore and bonding phase because it’s high fluidity, so it can be also speculated that compared to the pores between un-melted nuclei ore and bonding phase, void among sinter body sub-units played more important role on decrease of sinter strength.
4. Discussion
To clarify the action mechanism of MgO-bearing materials in sintering process, SEM-EDS analysis of NO. 1 to NO. 3 samples in the bonding strength tests were performed. Figure 11 shows the Microstructure of bonding phase using different MgO-bearing materials. Table 4 shows some examples of energy spectrum analysis results of the bonding phase. In general, all the bonding phases were mainly composed by CF (Calcium Ferrite), M (Magnetite or Magnetite solid solution), S (Slag) and P (Pores), and by comparison, two types of CF existed, CF1 contained high MgO content and low SiO2 content, CF2 contained low MgO content and high SiO2 content, in addition, two types of M also existed, M1 contained low MgO content and M2 contained high MgO content.
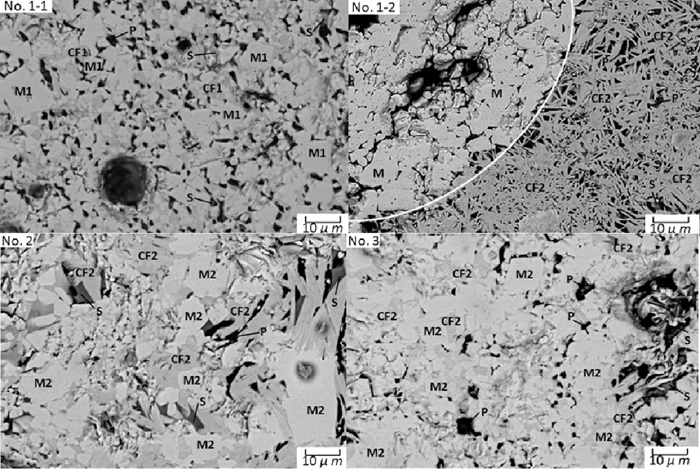
Table 4. Energy spectrum analysis results of the bonding phase (mass%).
| MgO-bearing materials | Bonding phase | O mass% | Si mass% | Mg mass% | Ca mass% | Fe mass% | Total mass% |
|---|
| NO. 1- Dolomite | CF2 | 29.1 | 5.9 | 0 | 19.4 | 45.6 | 100.0 |
| CF1 | 28.1 | 1.5 | 2.7 | 7.0 | 60.7 | 100.0 |
| M1 | 31.1 | 0 | 3.9 | 1.5 | 63.5 | 100.0 |
| NO. 2- L-Dolomite | CF2 | 29.7 | 4.2 | 0.8 | 12.2 | 53.1 | 100.0 |
| M2 | 28.8 | 0 | 6.7 | 2.1 | 62.4 | 100.0 |
| NO. 3- Serpentine | CF2 | 31.7 | 3.1 | 0.7 | 12.4 | 52.1 | 100.0 |
| M2 | 30.2 | 0 | 5.0 | 1.9 | 62.9 | 100.0 |
Fluidity of primary bonding phase at the outer of each sinter body sub-unit largely determined the size and amount of void among sinter body sub-units, thus the strength of sinter. For the high basicity sinter, the main bonding phase was calcium ferrite or SFCA, so the viscosity of calcium ferrite or SFCA determined the fluidity of bonding phase to a large extent. From Table 4, it could be found that compared to CF2 in the bonding phase using serpentine, CF1 contained higher MgO content, which resulting to higher viscosity of CF1. So based on above results and analysis, it can be speculated that the main reason decreasing the fluidity and bonding capacity of bonding phase using dolomite was the large MgO content in the calcium ferrite.
4.1. Action Mechanism of Dolomite in Sintering Process
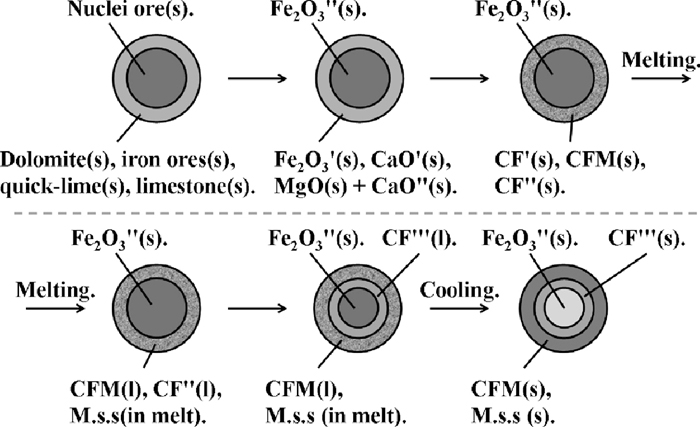
As shown in Fig. 10, the bonding phase using dolomite was composed by two parts, primary bonding phase and secondary bonding phase which were analyzed as NO. 1-1 and NO. 1-2 in Fig. 11 and Table 4 respectively. The formation process of primary bonding phase composed by CF1, M1, S and P and secondary bonding phase composed by CF2, S and P were influenced by the action mechanism of dolomite in the sintering process. The action mechanism of dolomite in the sintering process shown in Fig. 12 includes four steps.
First step: thermal decomposition
|
CaCO
3
(s)→CaO'(s)+
CO
2
(g)
| (2) |
|
CaCO
3
⋅
MgCO
3
(s)→MgO(s)+CaO''(s)+
CO
2
(g)
| (3) |
Second step: solid reaction
|
CaO'(s)+
Fe
2
O
3
'(s)→CF'(s)
| (4) |
|
MgO(s)+CaO''(s)+CF'(s)→CFM(s)+CF''(s)
| (5) |
Third step: formation of primary bonding phase
|
MgO(in melt) + CaO''(in melt) + Fe
2
O
3
'(in melt)
+ CF'(l)→CFM(l) + CF''(l)
| (6) |
|
MgO(in melt) + FeOx(in melt)→M.s.s(in melt)
| (7) |
Fourth step: formation of secondary bonding phase
|
CF'(l) +CF''(l) +
Fe
2
O
3
''(s)→CF'''(l)
| (8) |
Firstly, by using TG/DTA in N2 atmosphere, limestone decomposed into CaO' and CO2 at 819°C, and dolomite decomposed into MgO, CaO'' and CO2 at 804°C and 860°C respectively, which were similar to other literature data.4)
Secondly, CaO' from quick lime and limestone reacted with Fe2O3' from adhesive powder forming CF' (calcium ferrite), then MgO and CaO'' at the outer of dolomite reacted with CF' forming CFM (calcium ferrite with MgO) and CF'' (calcium ferrite) by solid reaction. Due to the poor dynamic condition, especially for the big size dolomite, solid reaction was insufficient, so part of dolomite was still exited as MgO and CaO'' before melt formation. In addition, according to the research results of Higuchi et al.,9) MgO also reacted with the FeOx forming dense M.s.s (Magnetite solid solution) by solid reaction at low temperature. But in this research, no dense M.s.s was discovered. The possible reason was that, the particle size of dolomite used in this bonding strength tests was very fine, below 0.15 mm, moreover, the reaction temperature of dolomite with calcium ferrite was about 1150°C, much lower than that of dolomite with iron ore, so nearly all the fine dolomite fused into the calcium ferrite firstly, then react with other materials, the formation reaction of dense M.s.s by solid reaction was poor.
Thirdly, with the formation of liquid phase, MgO and CaO'' at the inner of dolomite fused into melt and reacted with Fe2O3 (in melt) and CF' forming CF'' and CFM which has high melt-down temperature and high viscosity.14) In addition, MgO and FeOx in melt reacted with each other and formed M.s.s.
Finally, CF (including CF' and CF'') nearby the nuclei ore reacted with the un-melted particle and formed secondary liquid phase CF''', moreover, the low fluidity of primary liquid phase promoted the reaction between CF with nuclei ore to a great extent. So as shown in NO. 1 in Fig. 10, secondary bonding phase (B2) was observed clearly.
By the way, the effect of SiO2 and Al2O3 mainly from iron ores in adhesive powder were not discussed clearly in this study, it is because in this test, the occurrence states and proportion of SiO2 and Al2O3 were constant, which influenced the reaction process of MgO in the sintering process lightly.
In addition, the action mechanism of light burned dolomite in sintering process was similar to dolomite, the difference between light burned dolomite with dolomite was light burned dolomite had lower solid reaction temperature, which leading to more CF formation, thus higher fluidity of bonding phase. In addition, MgO and CaO had higher separation degree in light burned dolomite, which led to higher separation degree of MgO and CF', so as shown in NO. 2 in Fig. 11 and Table 4, bonding phase using light burned dolomite was composed by CF2, M2, S and P. Compared to CF1 and M1 in dolomite bonding phase, CF2 contained lower MgO content, M2 contained higher MgO content, and as mentioned above, calcium ferrite containing lower MgO content had higher fluidity and could bond the sinter body sub-units together better. Moreover, the light burning process maybe also led to different activity of MgO and CaO, which should be researched further later.
4.2. Action Mechanism of Serpentine in Sintering Process
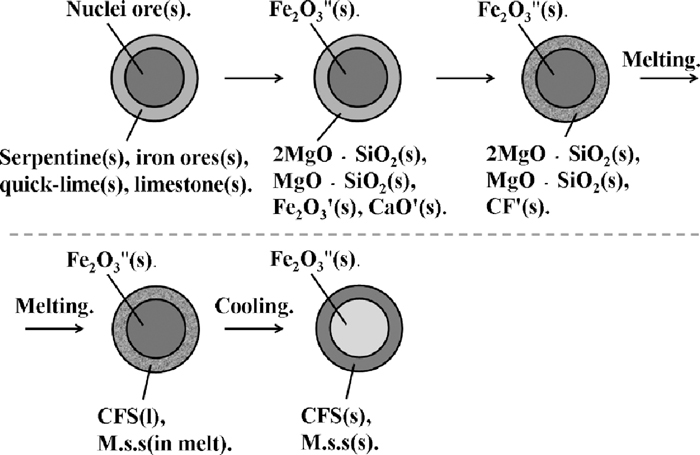
The bonding phase using serpentine was analyzed as NO. 3 in Fig. 11 and Table 4. The formation process of bonding phase composed by CF2, M2, S and P was influenced by the action mechanism of serpentine in the sintering process. The action mechanism of serpentine in the sintering process shown in Fig. 13 includes three steps.
First step: thermal decomposition
|
3MgO⋅
2SiO
2
⋅
2H
2
O(s) → 2MgO⋅
SiO
2
(s)
+ MgO⋅
SiO
2
(s) + 2H
2
O(g)
| (9) |
|
CaC
O
3
(s)
→CaO'(s) + C
O
2
(g)
| (10) |
Second step: solid reaction
|
CaO'(s)
+Fe
2
O
3
'(s)→CF'(s)
| (11) |
Third step: formation of primary bonding phase
|
2MgO⋅
SiO
2
(in melt) + MgO⋅
SiO
2
(in melt) + FeOx(in melt)
+ CF'(l) → M.s.s(in melt) + CFS(l)
| (12) |
Firstly, by using TG/DTA in N2 atmosphere, serpentine decomposed into 2MgO·SiO2, SiO2 and H2O at around 668°C, then free SiO2 reacted with 2MgO·SiO2 and formed MgO·SiO2 at around 836°C, and limestone also decomposed into CaO' and CO2 at 819°C.
Secondly, CaO' from quick lime and limestone reacted with Fe2O3' from iron ore adhesive powder forming CF'.
Thirdly, 2MgO·SiO2 and MgO·SiO2 fused into CF' and reacted with FeOx and CF' forming M.s.s and CFS (Fe2O3–CaO–SiO2 eutectic mixture) or SFCA.
Compared to calcium ferrite bonding phase using dolomite, low MgO content exited in calcium ferrite using serpentine, and according to research of Machida,11) below 1400°C, increasing MgO content in the calcium ferrite could increase the viscosity of liquid phase, and this tendency could enhance with the temperature decrease. For the high basicity sinter, calcium ferrite bonding phase was the main bonding phase which played the most important role on the liquid phase fluidity and sinter strength. In this study, the fluidity and bonding strength tests were finished at 1280°C, so bonding phase using serpentine had high fluidity, thus high bonding strength of sinter, and all of these were on account of the low MgO content in the calcium ferrite.
Based on above results, the most important thing to replace serpentine with dolomite or other MgO-bearing materials was decreasing the MgO content in the calcium ferrite. Increasing the solid solubility of MgO in magnetite, decreasing the dispersity of MgO in sinter bed, increasing the separation degree of MgO and CaO, and decreasing the reaction amount of MgO might be effective.
5. Sinter Pot Tests
To inspect the effectiveness and feasibility of the technique direction mentioned above, sinter pot tests were conducted. The sinter pot having an inner diameter of 200 mm and a height of 700 mm was used. Raw mixture having a weight of about 40 kg was granulated with adding water in a drum mixer to have moisture of 7.5 mass%, then sintered in the sinter pot under a suction of 11 kPa. Sinter cake was dropped from a height of 2 m for 5 times after sintering, and sinter over 5 mm was treated as sinter product. Table 5 shows the blending conditions of raw mixtures, MS of dolomite used in the sinter pot tests, and chemical composition of the sinter. Base-A blending mixture was demonstrated as high-SiO2 sinter condition with using blending ores, which mixed by different kinds of materials, such as Australian limonite fine ore, Brazilian hematite fine ore or Chilean magnetite concentrate, serpentine, dolomite, quick lime and limestone. In addition, sinter return and fuel were also used in a certain proportion routinely, the basicity and SiO2 content of Base-A sinter were 1.85 and 5.10 mass% respectively. In Base-B, all the serpentine was replaced by dolomite with mean size of 0.27 mm, and decreased the limestone content appropriately, then the basicity and SiO2 content of Base-B sinter were 1.85 and 4.65 mass% respectively. Scheme 1 (Sch 1) to Scheme 5 (Sch 5) were adjusted based on Base-B at the same basicity and SiO2 content. In Sch 1, mixing dolomite (4.5 mass% of sinter mixture, mean size of 0.27 mm) with Chilean magnetite concentrate (3.0 mass% of sinter mixture) and some water (0.28 kg) in a square iron box by manual mixing operation for about 5 min before granulation, for the purpose of improving the contact condition of MgO and Fe3O4, thus increasing the solid solubility of MgO in the magnetite and decreasing the MgO content in the calcium ferrite, and the mean size and moisture of the materials after mixing were 0.3 mm and 7.8 mass%. Moreover, Increasing the particle size of dolomite could decrease the dispersity of MgO in the sinter bed, but too much bigger particle size of dolomite maybe also restrict the performance of CaO in dolomite, so in Sch 2 and Sch 3, dolomite with bigger mean sizes were used, aiming to achieve the optimal particle size distribution of dolomite in sintering process. In Sch 4, dolomite was replaced by light burned dolomite, aiming to test the effect of increasing the separation degree of MgO and CaO in the dolomite on sinter strength. In Sch 5, dolomite was replaced by big particle size magnesia, aiming to test the performance of decreasing the reaction amount of MgO in sintering process.
Table 5. Blending condition of raw mixtures, MS of dolomite and chemical composition of sinter in sinter pot tests (mass%).
| | Base-A | Base-B | Sch 1 | Sch 2 | Sch 3 | Sch 4 | Sch 5 |
|---|
| Dolomite | mass% | 2.6 | 4.6 | 4.5 | 4.6 | 4.6 | 0 | 0 |
| L-Dolomite | mass% | 0 | 0 | 0 | 0 | 0 | 2.5 | 0 |
| Serpentine | mass% | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Magnesia | mass% | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 |
| Quick lime | mass% | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 |
| Limestone | mass% | 3.6 | 1.3 | 1.4 | 1.3 | 1.3 | 1.5 | 4.1 |
| Sinter return | mass% | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 |
| Fuel | mass% | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Iron ores | mass% | 57.5 | 58.7 | 58.7 | 58.7 | 58.7 | 60.6 | 59.6 |
| Total | mass% | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| MS of Dolomite | mm | – | 0.27 | 0.27 | 1.00 | 1.90 | – | – |
| Chemical composition and basicity of sinter |
| TFe | mass% | 56.04 | 56.99 | 56.97 | 56.99 | 56.99 | 57.07 | 57.03 |
| SiO2 | mass% | 5.10 | 4.65 | 4.65 | 4.65 | 4.65 | 4.65 | 4.65 |
| CaO | mass% | 9.45 | 8.60 | 8.61 | 8.60 | 8.60 | 8.59 | 8.61 |
| Al2O3 | mass% | 1.69 | 1.71 | 1.70 | 1.71 | 1.71 | 1.67 | 1.69 |
| MgO | mass% | 1.60 | 1.60 | 1.60 | 1.60 | 1.60 | 1.59 | 1.60 |
| Basicity | – | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 |
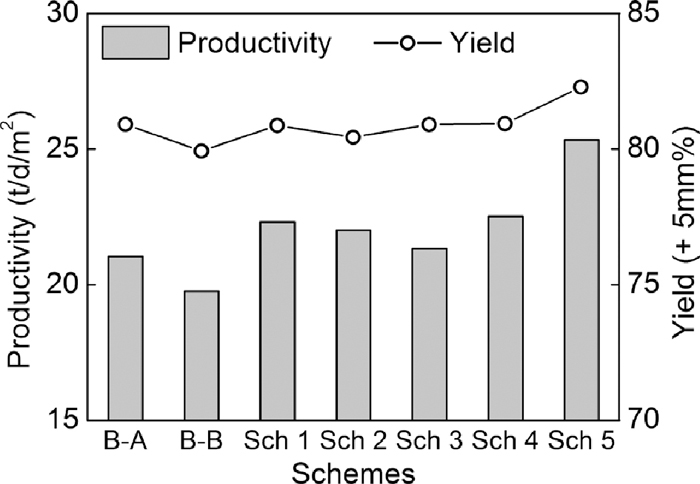
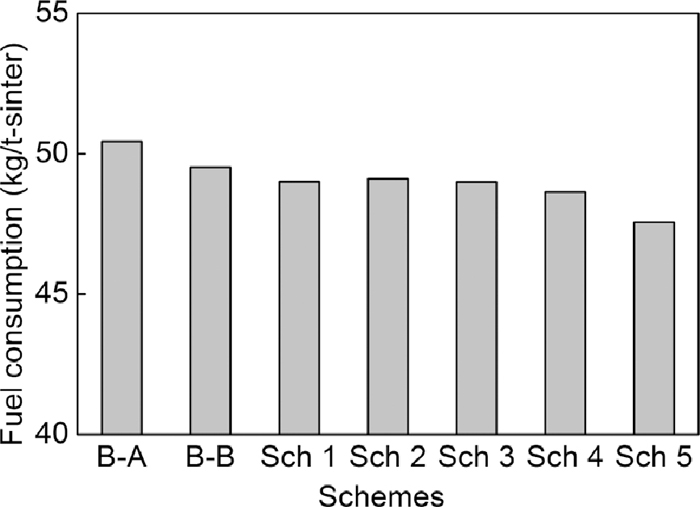
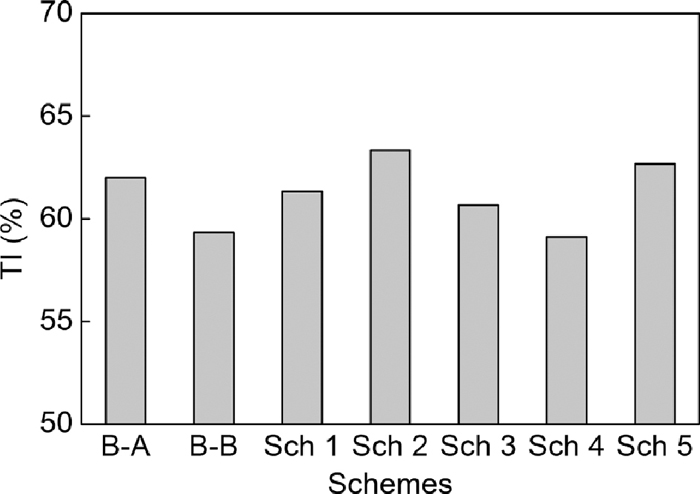
Figures 14, 15 and 16 show the sinter pot tests results. Compared to Base-A, tumbler strength, productivity and yield decreased obviously, and fuel consumption decreased less in the case of changing serpentine with dolomite in Base-B. Compared to Base-A, low SiO2 content (4.65 mass%) of Base-B led to low flux addition in the case of same basicity of 1.85, thus low fluidity of liquid phase and low strength of sinter, but the TFe content of sinter increased and fuel consumption decreased. Based on Base-B, in the same SiO2 content (4.65 mass%) condition, tumbler strength, productivity, yield increased in the case of mixing dolomite with magnetite concentrate in scheme 1, using bigger dolomite (1.00 mm and 1.90 mm) in scheme 2 and scheme 3, using light burned dolomite in scheme 4 and using big particle size magnesia (+ 6.3 mm) in scheme 5. In addition, fuel consumption decreased less in scheme 4 and decreased obviously in scheme 5 owing to the lower loss of ignition of light burned dolomite and magnesia.
6. Conclusions
In order to understand the action mechanism and the effective usage of dolomite in the sintering process aiming to produce high grade and low SiO2 sinter, solid reaction, fluidity of liquid phase, bonding strength and sinter pot tests were conducted, in addition, microstructure and energy spectrum were analyzed. The following conclusions were obtained.
(1) Reaction temperature of dolomite with calcium ferrite was lower than that with limonite iron ore about 155°C, even lower than the melting temperature of calcium ferrite.
(2) Fluidity and bonding capacity of bonding phase using dolomite were lower than that using light burned dolomite and serpentine owing to the higher MgO content in the calcium ferrite and formation of secondary bonding phase.
(3) Action mechanism of dolomite in sintering process involving two formation of original and secondary bonding phase was proposed. It mainly included 4 steps, thermal decomposition, solid reaction, formation of original bonding phase containing CFM and M.s.s, formation of secondary bonding phase. Dolomite mainly played it’s role in the third step in which high viscosity CFM (calcium ferrite with MgO) and magnetite solid solution formed.
(4) Dolomite mixed with magnetite concentrate before granulation kept good sintering indexes. Improving contact condition of MgO and FeOx could increase the solid solubility of MgO in the magnetite, thus decreased the content of MgO in calcium ferrite, and then increased the fluidity of bonding phase and strength of sinter.
(5) Dolomite with mean size of 1 mm showed good performance in sinter pot tests. Under the precondition didn’t affect the reaction rate of CaO, increasing the particle size of dolomite could decrease the dispersity of MgO in the sinter mixture, thus decreased the negative influence of MgO on the fluidity and bonding capacity of bonding phase.
Acknowledgement
The financial support of the National Natural Science Foundation of China (U1260202), and Fundamental Research Funds for the Central Universities (FRF-MP-12-003B) is gratefully acknowledged.
References
- 1) T. Maeda, K. Nishioka and M. Shimizu: ISIJ Int., 49 (2009), 625.
- 2) J. He: ISIJ Int., 51 (2011), 696.
- 3) Y. Hida and N. Nosaka: Tetsu-to-Hagané, 78 (1992), 960.
- 4) E. Kasai, Y. Waseda and M. V. Ramos: Tetsu-to-Hagané, 83 (1997), 539.
- 5) E. Kasai, Y. Sakano, T. Kawaguchi and T. Nakamura: ISIJ Int., 40 (2000), 857.
- 6) S. C. Panigrahy, M. Rigaud and J. Dilewijns: Ironmaking Steelmaking, 11 (1984), 246.
- 7) S. C. Panigrahy, A. J. Rigaud and J. Dilewijns: Steel Res., 56 (1985), 35.
- 8) Q. Li, Z. Huang, T. Jiang, Y. Yang and G. Li: Iron Steel., 41 (2006), 10.
- 9) K. Higuchi, T. Tanaka and T. Sato: ISIJ Int., 47 (2007), 669.
- 10) L. H. Hsien and J. A. Whiteman: ISIJ Int., 33 (1993), 462.
- 11) M. Matsumura, M. Hoshi and T. Kawaguchi: ISIJ Int., 45 (2005), 594.
- 12) S. Wu, Y. Liu and J. Du: J. Univ. Sci. Technol. Beijing, 24 (2002), 254.
- 13) S. Wu, J. Du and H. Ma: J. Univ. Sci. Technol. Beijing, 27 (2005), 291.
- 14) S. Machida, K. Nushiro, K. Ichikawa, H. Noda and H. Sakai: ISIJ Int., 45 (2005), 513.